Antenatal corticosteroids and perinatal outcome in late fetal growth restriction: analysis of prospective cohort
- PMID: 36412975
- PMCID: PMC10108243
- DOI: 10.1002/uog.26127
Antenatal corticosteroids and perinatal outcome in late fetal growth restriction: analysis of prospective cohort
Abstract
Objective: To evaluate the role of antenatal administration of corticosteroids for fetal lung maturation on the short-term perinatal outcome of pregnancy complicated by late fetal growth restriction (FGR).
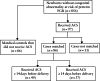
Methods: This cohort study was a secondary analysis of a multicenter prospective observational study, the TRUFFLE-2 feasibility study, conducted between 2017 and 2018 in 33 European perinatal centers. The study included women with a singleton pregnancy from 32 + 0 to 36 + 6 weeks of gestation with a fetus considered at risk for FGR, defined as estimated fetal weight (EFW) and/or fetal abdominal circumference < 10th percentile, or umbilicocerebral ratio (UCR) ≥ 95th percentile or a drop of more than 40 percentile points in abdominal circumference measurement from the 20-week scan. For the purposes of the current study, we identified women who received a single course of steroids to improve fetal lung maturation before delivery. Each exposed pregnancy was matched with one that did not receive antenatal corticosteroids (ACS) (control), based on gestational age at delivery and birth weight. The primary adverse outcome was a composite of abnormal condition at birth, major neonatal morbidity or perinatal death.
Results: A total of 86 pregnancies that received ACS were matched to 86 controls. The two groups were similar with respect to gestational age (33.1 vs 33.3 weeks), EFW (1673 vs 1634 g) and UCR (0.68 vs 0.62) at inclusion, and gestational age at delivery (35.5 vs 35.9 weeks) and birth weight (1925 vs 1948 g). No significant differences were observed between the exposed and non-exposed groups in the incidence of composite adverse outcome (28% vs 24%; P = 0.73) or any of its elements.
Conclusion: The present data do not show a beneficial effect of steroids on short-term outcome of fetuses with late FGR. © 2023 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: antenatal corticosteroids; fetal growth restriction; fetal lung maturation; late preterm.
© 2023 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Figures

Similar articles
-
Fetal cerebral blood-flow redistribution: analysis of Doppler reference charts and association of different thresholds with adverse perinatal outcome.Ultrasound Obstet Gynecol. 2021 Nov;58(5):705-715. doi: 10.1002/uog.23615. Ultrasound Obstet Gynecol. 2021. PMID: 33599336 Free PMC article.
-
Fetal cerebral Doppler changes and outcome in late preterm fetal growth restriction: prospective cohort study.Ultrasound Obstet Gynecol. 2020 Aug;56(2):173-181. doi: 10.1002/uog.22125. Ultrasound Obstet Gynecol. 2020. PMID: 32557921
-
Management of late-onset fetal growth restriction: pragmatic approach.Ultrasound Obstet Gynecol. 2023 Jul;62(1):106-114. doi: 10.1002/uog.26190. Ultrasound Obstet Gynecol. 2023. PMID: 36864542
-
Role of umbilicocerebral and cerebroplacental ratios in prediction of perinatal outcome in FGR pregnancies.Arch Gynecol Obstet. 2022 Jun;305(6):1383-1392. doi: 10.1007/s00404-021-06268-4. Epub 2021 Oct 2. Arch Gynecol Obstet. 2022. PMID: 34599678 Free PMC article. Review.
-
Fetal Growth Restriction: A Comprehensive Review of Major Guidelines.Obstet Gynecol Surv. 2023 Nov;78(11):690-708. doi: 10.1097/OGX.0000000000001203. Obstet Gynecol Surv. 2023. PMID: 38134339 Review.
Cited by
-
Antenatal Corticosteroids in Early and Late Fetal Growth Restriction.J Clin Med. 2025 Jul 9;14(14):4876. doi: 10.3390/jcm14144876. J Clin Med. 2025. PMID: 40725567 Free PMC article. Review.
-
Causal Relationships Between Leukocyte Subsets and Adverse Fetal Outcomes: A Mendelian Randomization Study.Mediators Inflamm. 2024 Dec 26;2024:6349687. doi: 10.1155/mi/6349687. eCollection 2024. Mediators Inflamm. 2024. PMID: 39748887 Free PMC article.
-
Maternal MitoQ Treatment Is Protective Against Programmed Alterations in CYP Activity Due to Antenatal Dexamethasone.Pharmaceutics. 2025 Feb 22;17(3):285. doi: 10.3390/pharmaceutics17030285. Pharmaceutics. 2025. PMID: 40142951 Free PMC article.
-
Outcome of Preterm Neonates > 32 Weeks Gestation in Relation to Three-Tiered Fetal Heart Rate Categorization.Medicina (Kaunas). 2025 Jun 28;61(7):1171. doi: 10.3390/medicina61071171. Medicina (Kaunas). 2025. PMID: 40731801 Free PMC article.
-
Understanding a Potential Role for the NLRP3 Inflammasome in Placenta-Mediated Pregnancy Complications.Am J Reprod Immunol. 2025 Apr;93(4):e70077. doi: 10.1111/aji.70077. Am J Reprod Immunol. 2025. PMID: 40260875 Free PMC article. Review.
References
-
- Piazze JJ, Maranghi L, Nigro G, Rizzo G, Cosmi EV, Anceschi MM. The effect of glucocorticoid therapy on fetal lung maturity indices in hypertensive pregnancies. Obstet Gynecol 1998; 92: 220–225. - PubMed
-
- Soregaroli M, Bonera R, Danti L, Dinolfo D, Taddei F, Valcamonico A, Frusca T. Prognostic role of umbilical artery Doppler velocimetry in growth‐restricted fetuses. J Matern Fetal Neonatal Med 2002; 11: 199–203. - PubMed
-
- Sterne G, Shields LE, Dubinsky TJ. Abnormal fetal cerebral and umbilical Doppler measurements in fetuses with intrauterine growth restriction predicts the severity of perinatal morbidity. J Clin Ultrasound 2001; 29: 146–151. - PubMed
-
- Torrance HL, Mulder EJ, Brouwers HA, Van Bel F, Visser GH. Respiratory outcome in preterm small for gestational age fetuses with or without abnormal umbilical artery Doppler and/or maternal hypertension. J Matern Fetal Neonatal Med 2007; 20: 613–621. - PubMed

