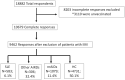
Safety and tolerance of vaccines against SARS-CoV-2 infection in systemic lupus erythematosus: results from the COVAD study
- PMID: 36413073
- PMCID: PMC10321116
- DOI: 10.1093/rheumatology/keac661
Safety and tolerance of vaccines against SARS-CoV-2 infection in systemic lupus erythematosus: results from the COVAD study
Abstract
Objective: To determine COVID-19 vaccine-related adverse events (AEs) in the seven-day post-vaccination period in patients with SLE vs autoimmune rheumatic diseases (AIRDs), non-rheumatic autoimmune diseases (nrAIDs), and healthy controls (HC).
Methods: Data were captured through the COVID-19 Vaccination in Autoimmune Diseases (COVAD) questionnaire (March-December 2021). Multivariable regression models accounted for age, gender, ethnicity, vaccine type and background treatment.
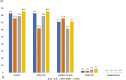
Results: Among 9462 complete respondents, 583 (6.2%) were SLE patients (mean age: 40.1 years; 94.5% females; 40.5% Asian; 42.9% Pfizer-recipients). Minor AEs were reported by 83.0% of SLE patients, major by 2.6%, hospitalization by 0.2%. AE and hospitalization frequencies were similar between patients with active and inactive SLE. Rashes were more frequent in SLE patients vs HC (OR; 95% CI: 1.2; 1.0, 1.5), chills less frequent in SLE vs AIRDs (0.6; 0.4, 0.8) and nrAIDs (0.5; 0.3, 0.8), and fatigue less frequent in SLE vs nrAIDs (0.6; 0.4, 0.9). Pfizer-recipients reported higher overall AE (2.2; 1.1, 4.2) and injection site pain (2.9; 1.6, 5.0) frequencies than recipients of other vaccines, Oxford/AstraZeneca-recipients more body ache, fever, chills (OR: 2.5, 3.0), Moderna-recipients more body ache, fever, chills, rashes (OR: 2.6, 4.3). Hospitalization frequencies were similar across vaccine types. AE frequencies were similar across treatment groups, although chills were less frequent in antimalarial users vs non-users (0.5; 0.3, 0.9).
Conclusion: While COVID-19 vaccination-related AEs were reported by four-fifths of SLE patients, those were mostly minor and comparable to AEs reported by healthy individuals, providing reassurance regarding COVID-19 vaccination safety in SLE.
Keywords: COVID-19; adverse events; rheumatology; systemic lupus erythematosus; vaccine.
© The Author(s) 2022. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures