Cell-Mediated Cytotoxicity in Lyme Arthritis
- PMID: 36413215
- PMCID: PMC10191881
- DOI: 10.1002/art.42408
Cell-Mediated Cytotoxicity in Lyme Arthritis
Abstract
Objective: Obliterative microvascular lesions are found in the synovial tissue of ~50% of patients with post-antibiotic Lyme arthritis (LA) and correlate with autoantibodies to certain vascular antigens. In this study, we identified lymphocytes with cytotoxic potential that may also mediate this feature of synovial pathology.
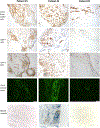
Methods: The cytotoxic potential of lymphocytes and their T cell receptor (TCR) Vβ gene usage were determined using samples of peripheral blood mononuclear cells (PBMCs) and synovial fluid mononuclear cells (SFMCs) from patients with antibiotic-responsive or post-antibiotic LA. Cell phenotypes were analyzed using flow cytometry and intracellular cytokine staining. Immunohistochemistry was performed on post-antibiotic synovial tissue samples.
Results: In SFMC and PBMC samples, the percentages of CD8+ T cells and double-negative T cells (primarily γδ T cells) were greater among 22 patients with post-antibiotic LA than in 14 patients with antibiotic-responsive LA. Moreover, CD8+ T cells and γδ T cells often expressed cytotoxic mediators, granzyme A/granzyme B, and perforin. The same 3 TCR Vβ segments were over-represented in both CD4+ T cells and CD8+ T cells in SFMC samples from post-antibiotic LA patients. In synovial tissue samples from 3 patients with post-antibiotic LA, CD8+ T cells intermixed with CD4+ T cells were seen around blood vessels, and 2 patients with microvascular damage had autoantibodies to vascular-associated antigens. One of these 2 patients, the one in whom cytotoxicity appeared to be active, had complement (C5b-9) deposition on obliterated vessels. Very few natural killer cells or γδ T cells were seen.
Conclusion: We propose that CD8+ T cell-mediated cytotoxicity, CD4+ T cell help, autoantibodies to vascular antigens, and complement deposition may each have a role in microvasculature damage in post-antibiotic LA.
© 2022 American College of Rheumatology.
Conflict of interest statement
The authors report no conflicts of interest.
Figures





Similar articles
-
Decline in the frequencies of Borrelia burgdorferi OspA161 175-specific T cells after antibiotic therapy in HLA-DRB1*0401-positive patients with antibiotic-responsive or antibiotic-refractory lyme arthritis.J Immunol. 2007 Nov 1;179(9):6336-42. doi: 10.4049/jimmunol.179.9.6336. J Immunol. 2007. PMID: 17947711
-
Correlation of Lyme Disease-Associated IgG4 Autoantibodies With Synovial Pathology in Antibiotic-Refractory Lyme Arthritis.Arthritis Rheumatol. 2018 Nov;70(11):1835-1846. doi: 10.1002/art.40566. Epub 2018 Sep 24. Arthritis Rheumatol. 2018. PMID: 29790305 Free PMC article.
-
Antibodies to endothelial cell growth factor and obliterative microvascular lesions in the synovium of patients with antibiotic-refractory lyme arthritis.Arthritis Rheumatol. 2014 Aug;66(8):2124-33. doi: 10.1002/art.38618. Arthritis Rheumatol. 2014. PMID: 24623727 Free PMC article.
-
Immunogenic HLA-DR-Presented Self-Peptides Identified Directly from Clinical Samples of Synovial Tissue, Synovial Fluid, or Peripheral Blood in Patients with Rheumatoid Arthritis or Lyme Arthritis.J Proteome Res. 2017 Jan 6;16(1):122-136. doi: 10.1021/acs.jproteome.6b00386. Epub 2016 Nov 7. J Proteome Res. 2017. PMID: 27726376 Free PMC article.
-
Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis.J Rheumatol. 1998 Aug;25(8):1466-71. J Rheumatol. 1998. PMID: 9712085
Cited by
-
Autoimmunity to synovial extracellular matrix proteins in patients with postinfectious Lyme arthritis.J Clin Invest. 2023 Sep 1;133(17):e161170. doi: 10.1172/JCI161170. J Clin Invest. 2023. PMID: 37471146 Free PMC article.
-
Variants in the Late Cornified Envelope Gene Locus Are Associated With Elevated T-helper 17 Responses in Patients With Postinfectious Lyme Arthritis.J Infect Dis. 2024 Aug 14;230(Supplement_1):S40-S50. doi: 10.1093/infdis/jiae164. J Infect Dis. 2024. PMID: 39140723 Free PMC article.
-
Wider recognition and greater understanding of postinfectious, antibiotic-refractory Lyme arthritis.J Clin Invest. 2024 Sep 3;134(17):e184109. doi: 10.1172/JCI184109. J Clin Invest. 2024. PMID: 39225104 Free PMC article.
-
Lyme Arthritis: A 50-Year Journey.J Infect Dis. 2024 Aug 14;230(Supplement_1):S1-S10. doi: 10.1093/infdis/jiae126. J Infect Dis. 2024. PMID: 39140724 Free PMC article.
-
Single cell immunophenotyping identifies CD8+ GZMK+ IFNG+ T cells as a key immune population in cutaneous Lyme disease.bioRxiv [Preprint]. 2025 Jun 12:2025.06.09.658661. doi: 10.1101/2025.06.09.658661. bioRxiv. 2025. PMID: 40661371 Free PMC article. Preprint.
References
-
- Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med 1987;107:725–31. - PubMed
-
- Steere AC, Levin RE, Molloy PJ, Kalish RA, Abraham JHIII, Liu NY, et al. Treatment of Lyme arthritis. Arthritis Rheum 1994;37:878–88. - PubMed
-
- Steere AC, Angelis SM. Therapy for Lyme arthritis; strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum 2006;54:3079–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

