Low-Value Prostate-Specific Antigen Test for Prostate Cancer Screening and Subsequent Health Care Utilization and Spending
- PMID: 36413364
- PMCID: PMC9682424
- DOI: 10.1001/jamanetworkopen.2022.43449
Low-Value Prostate-Specific Antigen Test for Prostate Cancer Screening and Subsequent Health Care Utilization and Spending
Abstract
Importance: Delivering low-value care can lead to unnecessary follow-up services and associated costs, and such care cascades have not been well examined in common clinical scenarios.
Objective: To evaluate the utilization and costs of care cascades of prostate-specific antigen (PSA) tests for prostate cancer screening, as the routine use of which among asymptomatic men aged 70 years and older is discouraged by multiple guidelines.
Design, setting, and participants: This cross-sectional study included men aged 70 years and older without preexisting prostate conditions enrolled in a Medicare Advantage plan during January 2016 to December 2018 with at least 1 outpatient visit. Medical billing claims data from the deidentified OptumLabs Data Warehouse were used. Data analysis was conducted from September 2020 to August 2021.
Exposures: At least 1 claim for low-value PSA tests for prostate cancer screening during the observation period.
Main outcomes and measures: Utilization of and spending on low-value PSA cancer screening and associated care cascades and the difference in overall health care utilization and spending among individuals receiving low-value PSA cancer screening vs those who did not, adjusting for observed characteristics using inverse probability of treatment weighting.
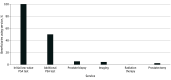
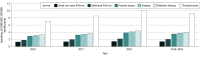
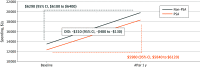
Results: Of 995 442 men (mean [SD] age, 78.0 [5.6] years) aged 70 years or older in a Medicare Advantage plan included in this study, 384 058 (38.6%) received a low-value PSA cancer screening. Utilization increased for each subsequent cohort from 2016 to 2018 (49 802 of 168 951 [29.4%] to 134 404 of 349 228 [38.5%] to 199 852 of 477 203 [41.9%]). Among those receiving initial low-value PSA cancer screening, 241 188 of 384 058 (62.8%) received at least 1 follow-up service. Repeated PSA testing was the most common, and 27 268 (7.1%) incurred high-cost follow-up services, such as imaging, radiation therapy, and prostatectomy. Utilization and spending associated with care cascades also increased from 2016 to 2018. For every $1 spent on a low-value PSA cancer screening, an additional $6 was spent on care cascades. Despite avoidable care cascades, individuals who received low-value PSA cancer screening were not associated with increased overall health care utilization and spending during the 1-year follow-up period compared with an unscreened population.
Conclusions and relevance: In this cross-sectional study, low-value PSA tests for prostate cancer screening remained prevalent among Medicare Advantage plan enrollees and were associated with unnecessary expenditures due to avoidable care cascades. Innovative efforts from clinicians and policy makers, such as payment reforms, to reduce initial low-value care and avoidable care cascades are warranted to decrease harm, enhance equity, and improve health care efficiency.
Conflict of interest statement
Figures
Similar articles
-
Assessment of Care Cascades Following Low-Value Prostate-Specific Antigen Testing Among Veterans Dually Enrolled in the US Veterans Health Administration and Medicare Systems.JAMA Netw Open. 2022 Dec 1;5(12):e2247180. doi: 10.1001/jamanetworkopen.2022.47180. JAMA Netw Open. 2022. PMID: 36520431 Free PMC article.
-
Downstream tests, treatments, and annual direct payments in older men cared for by primary care providers with high or low prostate-specific antigen screening rates using 100 percent Texas U.S. Medicare public insurance claims data: a retrospective cohort study.BMC Health Serv Res. 2016 Jan 15;16:17. doi: 10.1186/s12913-016-1265-1. BMC Health Serv Res. 2016. PMID: 26772175 Free PMC article.
-
Are there regional tendencies toward controversial screening practices? A study of prostate and breast cancer screening in a Medicare population.Cancer Epidemiol. 2017 Oct;50(Pt A):68-75. doi: 10.1016/j.canep.2017.07.015. Epub 2017 Aug 17. Cancer Epidemiol. 2017. PMID: 28822325
-
Prostate-Specific Antigen (PSA)-Based Population Screening for Prostate Cancer: An Economic Analysis.Ont Health Technol Assess Ser. 2015 May 1;15(11):1-37. eCollection 2015. Ont Health Technol Assess Ser. 2015. PMID: 26366237 Free PMC article. Review.
-
PSA Screening for Prostate Cancer: Why Saying No is a High-Value Health Care Choice.J Natl Compr Canc Netw. 2015 Dec;13(12):1566-74. doi: 10.6004/jnccn.2015.0182. J Natl Compr Canc Netw. 2015. PMID: 26656523 Review.
Cited by
-
Fiscal Impact of Expanded Medicare Coverage for GLP-1 Receptor Agonists to Treat Obesity.JAMA Health Forum. 2025 Apr 4;6(4):e250905. doi: 10.1001/jamahealthforum.2025.0905. JAMA Health Forum. 2025. PMID: 40279111 Free PMC article.
-
Time to de-implementation of low-value cancer screening practices: a narrative review.BMJ Qual Saf. 2025 Jul 18;34(8):547-555. doi: 10.1136/bmjqs-2025-018558. BMJ Qual Saf. 2025. PMID: 40393787 Free PMC article. Review.
-
Prostate-specific antigen testing rates in high-risk populations: results from the All of Us Research Program.Cancer Causes Control. 2024 Mar;35(3):509-521. doi: 10.1007/s10552-023-01807-7. Epub 2023 Oct 25. Cancer Causes Control. 2024. PMID: 37878135 Free PMC article.
-
An overview of utilizing artificial intelligence in localized prostate cancer imaging.Expert Rev Med Devices. 2025 Apr;22(4):293-310. doi: 10.1080/17434440.2025.2477601. Epub 2025 Mar 19. Expert Rev Med Devices. 2025. PMID: 40056148 Review.
-
Projected Savings From Reducing Low-Value Services in Medicare.JAMA Health Forum. 2025 Aug 1;6(8):e253050. doi: 10.1001/jamahealthforum.2025.3050. JAMA Health Forum. 2025. PMID: 40748546 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

