Chatbot-Delivered Cognitive Behavioral Therapy in Adolescents With Depression and Anxiety During the COVID-19 Pandemic: Feasibility and Acceptability Study
- PMID: 36413390
- PMCID: PMC9683529
- DOI: 10.2196/40242
Chatbot-Delivered Cognitive Behavioral Therapy in Adolescents With Depression and Anxiety During the COVID-19 Pandemic: Feasibility and Acceptability Study
Abstract
Background: Symptoms of depression and anxiety, suicidal ideation, and self-harm have escalated among adolescents to crisis levels during the COVID-19 pandemic. As a result, primary care providers (PCPs) are often called on to provide first-line care for these youth. Digital health interventions can extend mental health specialty care, but few are evidence based. We evaluated the feasibility of delivering an evidence-based mobile health (mHealth) app with an embedded conversational agent to deliver cognitive behavioral therapy (CBT) to symptomatic adolescents presenting in primary care settings during the pandemic.
Objective: In this 12-week pilot study, we evaluated the feasibility of delivering the app-based intervention to adolescents aged 13 to 17 years with moderate depressive symptoms who were treated in a practice-based research network (PBRN) of academically affiliated primary care clinics. We also obtained preliminary estimates of app acceptability, effectiveness, and usability.
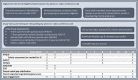
Methods: This small, pilot randomized controlled trial (RCT) evaluated depressive symptom severity in adolescents randomized to the app or to a wait list control condition. The primary end point was depression severity at 4-weeks, measured by the 9-item Patient Health Questionnaire (PHQ-9). Data on acceptability, feasibility, and usability were collected from adolescents and their parent or legal guardian. Qualitative interviews were conducted with 13 PCPs from 11 PBRN clinics to identify facilitators and barriers to incorporating mental health apps in treatment planning for adolescents with depression and anxiety.
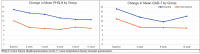
Results: The pilot randomized 18 participants to the app (n=10, 56%) or to a wait list control condition (n=8, 44%); 17 participants were included in the analysis, and 1 became ineligible upon chart review due to lack of eligibility based on documented diagnosis. The overall sample was predominantly female (15/17, 88%), White (15/17, 88%), and privately insured (15/17, 88%). Mean PHQ-9 scores at 4 weeks decreased by 3.3 points in the active treatment group (representing a shift in mean depression score from moderate to mild symptom severity categories) and 2 points in the wait list control group (no shift in symptom severity category). Teen- and parent-reported usability, feasibility, and acceptability of the app was high. PCPs reported preference for introducing mHealth interventions like the one in this study early in the course of care for individuals presenting with mild or moderate symptoms.
Conclusions: In this small study, we demonstrated the feasibility, acceptability, usability, and safety of using a CBT-based chatbot for adolescents presenting with moderate depressive symptoms in a network of PBRN-based primary care clinics. This pilot study could not establish effectiveness, but our results suggest that further study in a larger pediatric population is warranted. Future study inclusive of rural, socioeconomically disadvantaged, and underrepresented communities is needed to establish generalizability of effectiveness and identify implementation-related adaptations needed to promote broader uptake in pediatric primary care.
Trial registration: ClinicalTrials.gov NCT04603053; https://clinicaltrials.gov/ct2/show/NCT04603053.
Keywords: CBT; COVID-19; acceptability; adolescence; adolescent; adolescent depression; anxiety; chatbot; clinic; cognitive behavioral therapy; computer mediated; conversational agent; data; depression; digital health; feasibility; intervention; mental health; mobile health; pandemic; pediatric; primary care; psychiatry; relational conversational agent; self-harm; suicide; technology mediated; usability; youth.
©Ginger Nicol, Ruoyun Wang, Sharon Graham, Sherry Dodd, Jane Garbutt. Originally published in JMIR Formative Research (https://formative.jmir.org), 22.11.2022.
Conflict of interest statement
Conflicts of Interest: GN has received research support from Usona Institute (drug only), has served as principal or co-investigator on studies sponsored by Alkermes, Otsuka and LB Pharmaceuticals, and has received consulting fees from Alkermes, IngenioRx, Novartis and Sunovion. RW, SG, SD and JG have no conflicts of interest to disclose. AD, AR and SP are employees of WoeBot Health and were not involved in obtaining grant funding or IRB approval for this study, nor did they participate in data analyses. WoeBot Health provided app utilization data as reported in this manuscript.
Figures

References
-
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, Hedden SL, Crosby AE, Visser SN, Schieve LA, Parks SE, Hall JE, Brody D, Simile CM, Thompson WW, Baio J, Avenevoli S, Kogan MD, Huang LN, Centers for Disease Control and Prevention (CDC) Mental health surveillance among children--United States, 2005-2011. MMWR Suppl. 2013 May 17;62(2):1–35.su6202a1 - PubMed
-
- Asarnow JR, Jaycox LH, Duan N, LaBorde AP, Rea MM, Murray P, Anderson M, Landon C, Tang L, Wells KB. Effectiveness of a quality improvement intervention for adolescent depression in primary care clinics: a randomized controlled trial. JAMA. 2005 Jan 19;293(3):311–9. doi: 10.1001/jama.293.3.311.293/3/311 - DOI - PubMed
-
- McCarthy C. Anxiety in teens is rising: What's going on? American Academy of Pediatrics. 2019. [2020-09-10]. https://www.healthychildren.org/English/health-issues/conditions/emotion... .
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

