VpdC is a ubiquitin-activated phospholipase effector that regulates Legionella vacuole expansion during infection
- PMID: 36413498
- PMCID: PMC9860323
- DOI: 10.1073/pnas.2209149119
VpdC is a ubiquitin-activated phospholipase effector that regulates Legionella vacuole expansion during infection
Abstract
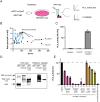
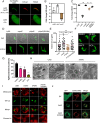
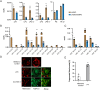
Intravacuolar pathogens need to gradually expand their surrounding vacuole to accommodate the growing number of bacterial offspring during intracellular replication. Here we found that Legionella pneumophila controls vacuole expansion by fine-tuning the generation of lysophospholipids within the vacuolar membrane. Upon allosteric activation by binding to host ubiquitin, the type IVB (Dot/Icm) effector VpdC converts phospholipids into lysophospholipids which, at moderate concentrations, are known to promote membrane fusion but block it at elevated levels by generating excessive positive membrane curvature. Consequently, L. pneumophila overproducing VpdC were prevented from adequately expanding their surrounding membrane, trapping the replicating bacteria within spatially confined vacuoles and reducing their capability to proliferate intracellularly. Quantitative lipidomics confirmed a VpdC-dependent increase in several types of lysophospholipids during infection, and VpdC production in transiently transfected cells caused tubulation of organelle membranes as well as mitochondria fragmentation, processes that can be phenocopied by supplying cells with exogenous lysophospholipids. Together, these results demonstrate an important role for bacterial phospholipases in vacuolar expansion.
Keywords: Legionella-containing vacuole; lysophospholipids; phospholipase A2; ubiquitin; vacuole expansion.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Dowhan W., “The role of phospholipids in cell function” in Advances in Lipobiology, Gross R. W., Ed. (JAI, 1997), vol. 2, pp. 79–107.
-
- Heringdorf D. M. z., “Lysophospholipids” in Encyclopedia of Molecular Pharmacology, Offermanns S., Rosenthal W., Eds. (Springer, Berlin, Heidelberg, 2008), pp. 710–716, 10.1007/978-3-540-38918-7_90. - DOI
-
- Yeagle P. L., Smith F. T., Young J. E., Flanagan T. D., Inhibition of membrane fusion by lysophosphatidylcholine. Biochemistry 33, 1820–1827 (1994). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

