3 Tesla magnetic resonance imaging in patients with cardiac implantable electronic devices: a single centre experience
- PMID: 36413601
- PMCID: PMC9935018
- DOI: 10.1093/europace/euac213
3 Tesla magnetic resonance imaging in patients with cardiac implantable electronic devices: a single centre experience
Abstract
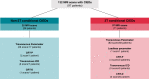
Aims: Three Tesla (T) magnetic resonance imaging (MRI) provides critical imaging information for many conditions. Owing to potential interactions of the magnetic field, it is largely withheld from patients with cardiac implantable electronic devices (CIEDs). Therefore, we assessed the safety of 3T MRI in patients with '3T MRI-conditional' and 'non-3T MRI-conditional' CIEDs.
Methods and results: We performed a retrospective single-centre analysis of clinically indicated 3T MRI examinations in patients with conventional pacemakers, cardiac resynchronization devices, and implanted defibrillators from April 2020 to May 2022. All CIEDs were interrogated and programmed before and after scanning. Adverse events included all-cause death, arrhythmias, loss of capture, inappropriate anti-tachycardia therapies, electrical reset, and lead or generator failure during or shortly after MRI. Changes in signal amplitude and lead impedance were systematically assessed. Statistics included median and interquartile range. A total of 132 MRI examinations were performed on a 3T scanner in 97 patients. Thirty-five examinations were performed in patients with 'non-3T MRI-conditional' CIEDs. Twenty-six scans were performed in pacemaker-dependent patients. No adverse events occurred during or shortly after MRI. P-wave or R-wave reductions ≥ 50 and ≥ 25%, respectively, were noted after three (2.3%) scans, all in patients with '3T MRI-conditional' CIEDs. Pacing and shock impedance changed by ± 30% in one case (0.7%). Battery voltage and stimulation thresholds did not relevantly change after MRI.
Conclusion: Pending verification in independent series, our data suggest that clinically indicated MRI scans at 3T field strength should not be withheld from patients with cardiac pacemakers or defibrillators.
Keywords: Cardiac implantable electronic devices; Implantable cardioverter defibrillator; Magnetic resonance imaging; Pacemakers; Tesla.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: Dr Fluschnik received a grant from Biotronik, all outside this submitted work. Dr Tahir has no disclosures to declare. Dr Erley has no disclosures to declare. Dr Müllerleile has no disclosures to declare. Dr Metzner received consultant fees from Medtronic, Biosense Webster and Lecture honoraria from Medtronic, Biosense Webster, Boston Scientific, Cardiofocus, Bayer. Dr Wenzel has no disclosures to declare. Dr Guerreiro has no disclosures to declare. Dr Adam has no disclosures to declare. Dr Blankenberg has received speakers fee from Medtronic, Pfizer, Roche, Novartis, SiemensDiagnostics (unrelated to the submitted work). Dr Kirchhof receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past, but not in the last three years (unrelated to the submitted work). Dr Kirchhof is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). Dr Tönnis has no disclosures to declare. Dr Nikorowitsch has no disclosures to declare.
Figures


References
-
- OECD (2022), Magnetic resonance imaging (MRI) exams (indicator). doi: 10.1787/1d89353f-en(Accessed on 20 January 2022). OECD; 2021. - DOI
-
- Sabzevari K, Oldman J, Herrey AS, Moon JC, Kydd AC, Manisty C. Provision of magnetic resonance imaging for patients with ‘MR-conditional’ cardiac implantable electronic devices: an unmet clinical need. Europace 2017;19:425–31. - PubMed
-
- Deshpande S, Kella D, Padmanabhan D. MRI In patients with cardiac implantable electronic devices: a comprehensive review. PACE—Pacing Clin Electrophysiol 2021;44(2):360–72. - PubMed
-
- Shellock FG. Biomedical implants and devices: assessment of magnetic field interactions with a 3.0-tesla MR system. J Magn Reson Imaging 2002;16:721–32. - PubMed
-
- Zbinden R, Wollmann C, Brachmann J, Michaelsen J, Steinwender C, Kovoor Pet al. Clinical safety of the ProMRI implantable cardioverter-defibrillator systems during head and lower lumbar magnetic resonance imaging at 3 T: results of the ProMRI 3T ENHANCED master study. Europace 2019;21(11):1678–85. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

