Cortical synapses of the world's smallest mammal: An FIB/SEM study in the Etruscan shrew
- PMID: 36413612
- PMCID: PMC10100312
- DOI: 10.1002/cne.25432
Cortical synapses of the world's smallest mammal: An FIB/SEM study in the Etruscan shrew
Abstract
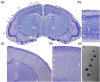
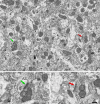
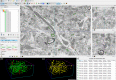
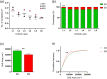
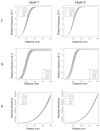
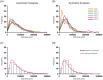
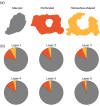
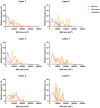
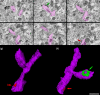
The main aim of the present study was to determine if synapses from the exceptionally small brain of the Etruscan shrew show any peculiarities compared to the much larger human brain. We analyzed the cortical synaptic density and a variety of structural characteristics of 7,239 3D reconstructed synapses, using using Focused Ion Beam/Scanning Electron Microscopy (FIB/SEM). We found that some of the general synaptic characteristics are remarkably similar to those found in the human cerebral cortex. However, the cortical volume of the human brain is about 50,000 times larger than the cortical volume of the Etruscan shrew, while the total number of cortical synapses in human is only 20,000 times the number of synapses in the shrew, and synaptic junctions are 35% smaller in the Etruscan shrew. Thus, the differences in the number and size of synapses cannot be attributed to a brain size scaling effect but rather to adaptations of synaptic circuits to particular functions.
Keywords: FIB-SEM; brain; cerebral cortex; electron microscopy; synaptic junction; ultrastructure.
© 2022 The Authors. The Journal of Comparative Neurology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Cortical organization in the Etruscan shrew (Suncus etruscus).J Neurophysiol. 2010 Nov;104(5):2389-406. doi: 10.1152/jn.00762.2009. Epub 2010 Jul 28. J Neurophysiol. 2010. PMID: 20668271
-
Cytoarchitecture, areas, and neuron numbers of the Etruscan shrew cortex.J Comp Neurol. 2012 Aug 1;520(11):2512-30. doi: 10.1002/cne.23053. J Comp Neurol. 2012. PMID: 22252518
-
Relative enlargement of the medial preoptic nucleus in the Etruscan shrew, the smallest torpid mammal.Sci Rep. 2022 Nov 3;12(1):18602. doi: 10.1038/s41598-022-22320-y. Sci Rep. 2022. PMID: 36329087 Free PMC article.
-
The neurobiology of Etruscan shrew active touch.Philos Trans R Soc Lond B Biol Sci. 2011 Nov 12;366(1581):3026-36. doi: 10.1098/rstb.2011.0160. Philos Trans R Soc Lond B Biol Sci. 2011. PMID: 21969684 Free PMC article. Review.
-
Etruscan shrew muscle: the consequences of being small.J Exp Biol. 2002 Aug;205(Pt 15):2161-6. doi: 10.1242/jeb.205.15.2161. J Exp Biol. 2002. PMID: 12110649 Review.
Cited by
-
Volume electron microscopy analysis of synapses in primary regions of the human cerebral cortex.Cereb Cortex. 2024 Aug 1;34(8):bhae312. doi: 10.1093/cercor/bhae312. Cereb Cortex. 2024. PMID: 39106175 Free PMC article.
-
Invariance of Mitochondria and Synapses in the Primary Visual Cortex of Mammals Provides Insight Into Energetics and Function.J Comp Neurol. 2024 Sep;532(9):e25669. doi: 10.1002/cne.25669. J Comp Neurol. 2024. PMID: 39291629
-
Tracing nerve fibers with volume electron microscopy to quantitatively analyze brain connectivity.Commun Biol. 2024 Jul 1;7(1):796. doi: 10.1038/s42003-024-06491-0. Commun Biol. 2024. PMID: 38951162 Free PMC article.
-
3D synaptic organization of layer III of the human anterior cingulate and temporopolar cortex.Cereb Cortex. 2023 Aug 23;33(17):9691-9708. doi: 10.1093/cercor/bhad232. Cereb Cortex. 2023. PMID: 37455478 Free PMC article.
-
Adult neurogenesis and "immature" neurons in mammals: an evolutionary trade-off in plasticity?Brain Struct Funct. 2024 Nov;229(8):1775-1793. doi: 10.1007/s00429-023-02717-9. Epub 2023 Oct 13. Brain Struct Funct. 2024. PMID: 37833544 Free PMC article. Review.
References
-
- Ascoli, G. A. , Alonso‐Nanclares, L. , Anderson, S. A. , Barrionuevo, G. , Benavides‐Piccione, R. , Burkhalter, A. , Buzsáki, G. , Cauli, B. , Defelipe, J. , Fairén, A. , Feldmeyer, D. , Fishell, G. , Fregnac, Y. , Freund, T. F. , Gardner, D. , Gardner, E. P. , Goldberg, J. H. , Helmstaedter, M. , Hestrin, S. , … Yuste, R. (2008). Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nature Reviews Neuroscience, 9, 557–568. 10.1038/nrn2402 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

