Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability
- PMID: 36413919
- PMCID: PMC9679038
- DOI: 10.1016/j.redox.2022.102535
Hemoglobin is an oxygen-dependent glutathione buffer adapting the intracellular reduced glutathione levels to oxygen availability
Abstract
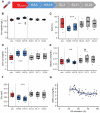
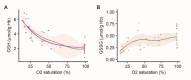
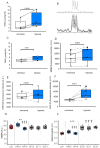
Fast changes in environmental oxygen availability translate into shifts in mitochondrial free radical production. An increase in intraerythrocytic reduced glutathione (GSH) during deoxygenation would support the detoxification of exogenous oxidants released into the circulation from hypoxic peripheral tissues. Although reported, the mechanism behind this acute oxygen-dependent regulation of GSH in red blood cells remains unknown. This study explores the role of hemoglobin (Hb) in the oxygen-dependent modulation of GSH levels in red blood cells. We have demonstrated that a decrease in Hb O2 saturation to 50% or less observed in healthy humans while at high altitude, or in red blood cell suspensions results in rising of the intraerythrocytic GSH level that is proportional to the reduction in Hb O2 saturation. This effect was not caused by the stimulation of GSH de novo synthesis or its release during deglutathionylation of Hb's cysteines. Using isothermal titration calorimetry and in silico modeling, we observed the non-covalent binding of four molecules of GSH to oxy-Hb and the release of two of them upon deoxygenation. Localization of the GSH binding sites within the Hb molecule was identified. Oxygen-dependent binding of GSH to oxy-Hb and its release upon deoxygenation occurred reciprocally to the binding and release of 2,3-bisphosphoglycerate. Furthermore, noncovalent binding of GSH to Hb moderately increased Hb oxygen affinity. Taken together, our findings have identified an adaptive mechanism by which red blood cells may provide an advanced antioxidant defense to respond to oxidative challenges immediately upon deoxygenation.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors of the manuscript REDOX-D-22-00013 have no conflict of interests to declare.
Figures




References
-
- Tsantes A.E., Bonovas S., Travlou A., Sitaras N.M. Redox imbalance, macrocytosis, and RBC homeostasis. Antioxidants Redox Signal. 2006;8:1205–1216. - PubMed
-
- Van Zwieten R., Verhoeven A.J., Roos D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic. Biol. Med. 2014;67:377–386. - PubMed
-
- Rossi R., Giustarini D., Milzani A., Dalle-Donne I. Membrane skeletal protein S-glutathionylation and hemolysis in human red blood cells. Blood Cells Mol. Dis. 2006;37:180–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

