Cerebellar GABA Change during Visuomotor Adaptation Relates to Adaptation Performance and Cerebellar Network Connectivity: A Magnetic Resonance Spectroscopic Imaging Study
- PMID: 36414012
- PMCID: PMC9581563
- DOI: 10.1523/JNEUROSCI.0096-22.2022
Cerebellar GABA Change during Visuomotor Adaptation Relates to Adaptation Performance and Cerebellar Network Connectivity: A Magnetic Resonance Spectroscopic Imaging Study
Abstract
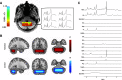
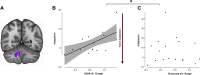
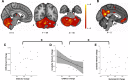
Motor adaptation is crucial for performing accurate movements in a changing environment and relies on the cerebellum. Although cerebellar involvement has been well characterized, the neurochemical changes in the cerebellum underpinning human motor adaptation remain unknown. We used a novel magnetic resonance spectroscopic imaging (MRSI) technique to measure changes in the inhibitory neurotransmitter GABA in the human cerebellum during visuomotor adaptation. Participants (n = 17, six female) used their right hand to adapt to a rotated cursor in the scanner, compared with a control task requiring no adaptation. We spatially resolved adaptation-driven GABA changes at the cerebellar nuclei and cerebellar cortex in the left and the right cerebellar hemisphere independently and found that simple right-hand movements increase GABA in the right cerebellar nuclei and decreases GABA in the left. When isolating adaptation-driven GABA changes, we found that GABA in the left cerebellar nuclei and the right cerebellar nuclei diverged, although GABA change from baseline at the right cerebellar nuclei was not different from zero at the group level. Early adaptation-driven GABA fluctuations in the right cerebellar nuclei correlated with adaptation performance. Participants showing greater GABA decrease adapted better, suggesting early GABA change is behaviorally relevant. Early GABA change also correlated with functional connectivity change in a cerebellar network. Participants showing greater decreases in GABA showed greater strength increases in cerebellar network connectivity. Results were specific to GABA, to adaptation, and to the cerebellar network. This study provides first evidence for plastic changes in cerebellar neurochemistry during motor adaptation. Characterizing these naturally occurring neurochemical changes may provide a basis for developing therapeutic interventions to facilitate human motor adaptation.SIGNIFICANCE STATEMENT Despite motor adaptation being fundamental to maintaining accurate movements, its neurochemical basis remains poorly understood, perhaps because measuring neurochemicals in the human cerebellum is technically challenging. Using a novel magnetic resonance spectroscopic imaging method, this study provides evidence for GABA changes in the left compared with the right cerebellar nuclei driven by both simple movement and motor adaptation. Although right cerebellar GABA changes were not significantly different from zero at the group level, the adaptation-driven GABA fluctuations in the right cerebellar nuclei correlated with adaptation performance and with functional connectivity change in a cerebellar network. These results show the first evidence for plastic changes in cerebellar neurochemistry during a cerebellar learning task. This provides the basis for developing therapeutic interventions that facilitate these naturally occurring changes to amplify cerebellar-dependent learning.
Keywords: GABA; cerebellum; functional connectivity; neuroimaging; spectroscopy; visuomotor adaptation.
Copyright © 2022 the authors.
Figures


References
-
- Albus JS (1971) A theory of cerebellar function. Math Biosci 10:25–61. 10.1016/0025-5564(71)90051-4 - DOI
-
- Andersson JLR, Jenkinson M, Smith S (2007) Non-linear registration aka spatial normalisation. FMRIB Technical Report TRO7JA2. Available at https://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
