Predictor factor for worse outcomes in kidney transplant recipients infected with coronavirus disease 2019: A systematic review and meta-analysis
- PMID: 36414181
- PMCID: PMC9675086
- DOI: 10.1016/j.trim.2022.101739
Predictor factor for worse outcomes in kidney transplant recipients infected with coronavirus disease 2019: A systematic review and meta-analysis
Abstract
Introduction: The pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a massive impact on the health sector, especially in patients with pre-existing comorbidities. This study aims to define the predictor factors for worse outcomes in kidney transplant patients infected with SARS-CoV-2 and affected by coronavirus disease 2019 (COVID-19). We have analyzed in these patients their prior medical history, their clinical symptoms, and their laboratory results.
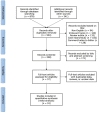
Method: We assessed outcomes of kidney transplant patients with confirmed COVID-19 until July 2021 from PubMed, Medline, Science Direct, Cochrane databases, EMBASE, Scopus, and EBSCO. We performed meta-analyses of nine published studies to estimate predictor factors. The analysis was analyzed by the Newcastle-Ottawa Scale (NOS) and then using the Review Manager 5.4 software.
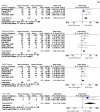
Result: Our analysis demonstrated that the most significant risk factors for the worse COVID-19 outcomes for kidney transplant patients included: age of 60 and older [MD 9.31(95% CI, 6.31-12.30), p < 0.0001, I2 = 76%], diabetic nephropathy [OR 2.13 (95% CI, 1.49-3.04), p < 0.0001, I2 = 76%], dyspnea [OR 4.53, (95% CI, 2.22-9.22), p < 0.0001, I2 = 76%], acute kidney injury (AKI) [OR 4.53 (95% CI, 1.10-5.21), p = 0.03, I2 = 58%], and some laboratory markers. Many patients had two or multiple risk factors in combination.
Conclusion: Age and several comorbidities were the most significant factors for COVID-19 outcomes for kidney transplant recipients.
Keywords: COVID-19; Kidney transplant; Meta-analysis; Outcome; Risk factor; Systematic review.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The author declares that he has no conflict of interest.
Figures





Similar articles
-
Measures implemented in the school setting to contain the COVID-19 pandemic.Cochrane Database Syst Rev. 2022 Jan 17;1(1):CD015029. doi: 10.1002/14651858.CD015029. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 May 2;5:CD015029. doi: 10.1002/14651858.CD015029.pub2. PMID: 35037252 Free PMC article. Updated.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article.
-
Interventions for palliative symptom control in COVID-19 patients.Cochrane Database Syst Rev. 2021 Aug 23;8(8):CD015061. doi: 10.1002/14651858.CD015061. Cochrane Database Syst Rev. 2021. PMID: 34425019 Free PMC article.
Cited by
-
Shifting perspectives in liver diseases after kidney transplantation.World J Hepatol. 2023 Jul 27;15(7):883-896. doi: 10.4254/wjh.v15.i7.883. World J Hepatol. 2023. PMID: 37547033 Free PMC article. Review.
-
A Case of Acute Kidney Injury Caused by Myoglobin Cast Nephropathy With Sars-Cov-2 Infection in a Living-Donor Kidney Transplant Recipient.Transplant Proc. 2023 May;55(4):1055-1058. doi: 10.1016/j.transproceed.2023.03.068. Epub 2023 Apr 6. Transplant Proc. 2023. PMID: 37208224 Free PMC article.
-
A review of the clinical characteristics and management of immunosuppressed patients living with HIV or solid organ transplants infected with SARS-CoV-2 omicron variants.Front Public Health. 2024 Feb 22;12:1327093. doi: 10.3389/fpubh.2024.1327093. eCollection 2024. Front Public Health. 2024. PMID: 38454994 Free PMC article. Review.
-
Clinical characteristics and outcomes in COVID-19 in kidney transplant recipients: a propensity score matched cohort study.Front Med (Lausanne). 2024 Apr 15;11:1350657. doi: 10.3389/fmed.2024.1350657. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38686364 Free PMC article.
References
-
- Bansal A., Kumar A., Bansal R., Maheshwari R., Chaturvedi S. The impact of comorbidities on clinical course and outcome, in kidney transplant recipients with COVID-19: a systematic review and analysis. Indian J. Transplant. 2020;14(4):275.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

