Multivalent Fucosides Targeting β-Propeller Lectins from Lung Pathogens with Promising Anti-Adhesive Properties
- PMID: 36414265
- PMCID: PMC9764287
- DOI: 10.1021/acschembio.2c00708
Multivalent Fucosides Targeting β-Propeller Lectins from Lung Pathogens with Promising Anti-Adhesive Properties
Abstract
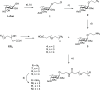
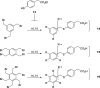
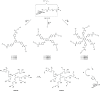
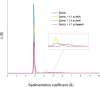
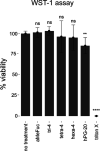
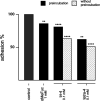
Fungal and bacterial pathogens causing lung infections often use lectins to mediate adhesion to glycoconjugates at the surface of host tissues. Given the rapid emergence of resistance to the treatments in current use, β-propeller lectins such as FleA from Aspergillus fumigatus, SapL1 from Scedosporium apiospermum, and BambL from Burkholderia ambifaria have become appealing targets for the design of anti-adhesive agents. In search of novel and cheap anti-infectious agents, we synthesized multivalent compounds that can display up to 20 units of fucose, the natural ligand. We obtained nanomolar inhibitors that are several orders of magnitude stronger than their monovalent analogue according to several biophysical techniques (i.e., fluorescence polarization, isothermal titration calorimetry, and bio-layer interferometry). The reason for high affinity might be attributed to a strong aggregating mechanism, which was examined by analytical ultracentrifugation. Notably, the fucosylated inhibitors reduced the adhesion of A. fumigatus spores to lung epithelial cells when administered 1 h before or after the infection of human lung epithelial cells. For this reason, we propose them as promising anti-adhesive drugs for the prevention and treatment of aspergillosis and related microbial lung infections.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Multivalent Fucosides with Nanomolar Affinity for the Aspergillus fumigatus Lectin FleA Prevent Spore Adhesion to Pneumocytes.Chemistry. 2018 Dec 20;24(72):19243-19249. doi: 10.1002/chem.201803602. Epub 2018 Nov 26. Chemistry. 2018. PMID: 30277619
-
Biochemical and structural studies of target lectin SapL1 from the emerging opportunistic microfungus Scedosporium apiospermum.Sci Rep. 2021 Aug 9;11(1):16109. doi: 10.1038/s41598-021-95008-4. Sci Rep. 2021. PMID: 34373510 Free PMC article.
-
Multivalent Glycomimetics with Affinity and Selectivity toward Fucose-Binding Receptors from Emerging Pathogens.Bioconjug Chem. 2018 Jan 17;29(1):83-88. doi: 10.1021/acs.bioconjchem.7b00616. Epub 2017 Dec 26. Bioconjug Chem. 2018. PMID: 29240403
-
Glycoconjugates and Glycomimetics as Microbial Anti-Adhesives.Trends Biotechnol. 2016 Jun;34(6):483-495. doi: 10.1016/j.tibtech.2016.01.004. Epub 2016 Feb 12. Trends Biotechnol. 2016. PMID: 26875976 Review.
-
Carbohydrates as future anti-adhesion drugs for infectious diseases.Biochim Biophys Acta. 2006 Apr;1760(4):527-37. doi: 10.1016/j.bbagen.2005.12.008. Epub 2006 Jan 18. Biochim Biophys Acta. 2006. PMID: 16564136 Review.
Cited by
-
Using next generation antimicrobials to target the mechanisms of infection.NPJ Antimicrob Resist. 2023;1(1):11. doi: 10.1038/s44259-023-00011-6. Epub 2023 Sep 22. NPJ Antimicrob Resist. 2023. PMID: 38686217 Free PMC article. Review.
-
The influence of tag sequence on recombinant humanized collagen (rhCol) and the evaluation of rhCol on Schwann cell behaviors.Regen Biomater. 2023 Oct 16;10:rbad089. doi: 10.1093/rb/rbad089. eCollection 2023. Regen Biomater. 2023. PMID: 38020236 Free PMC article.
-
Tandem-repeat lectins: structural and functional insights.Glycobiology. 2024 May 26;34(7):cwae041. doi: 10.1093/glycob/cwae041. Glycobiology. 2024. PMID: 38857376 Free PMC article.
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2021-2022.Mass Spectrom Rev. 2025 May-Jun;44(3):213-453. doi: 10.1002/mas.21873. Epub 2024 Jun 24. Mass Spectrom Rev. 2025. PMID: 38925550 Free PMC article. Review.
-
In Vivo Evidence on the Emerging Potential of Non-Digestible Oligosaccharides as Therapeutic Agents in Bacterial and Viral Infections.Nutrients. 2025 Mar 19;17(6):1068. doi: 10.3390/nu17061068. Nutrients. 2025. PMID: 40292455 Free PMC article. Review.
References
-
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. https://amr-review.org (accessed May 2016).
-
- Chopra I.; Schofield C.; Everett M.; O’Neill A.; Miller K.; Wilcox M.; Frère J. M.; Dawson M.; Czaplewski L.; Urleb U.; Courvalin P. Treatment of Health-Care-Associated Infections Caused by Gram-Negative Bacteria: A Consensus Statement. Lancet Infect. Dis. 2008, 8, 133–139. 10.1016/s1473-3099(08)70018-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

