Genetic Variants in ARHGEF6 Cause Congenital Anomalies of the Kidneys and Urinary Tract in Humans, Mice, and Frogs
- PMID: 36414417
- PMCID: PMC10103091
- DOI: 10.1681/ASN.2022010050
Genetic Variants in ARHGEF6 Cause Congenital Anomalies of the Kidneys and Urinary Tract in Humans, Mice, and Frogs
Abstract
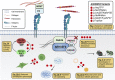
Background: About 40 disease genes have been described to date for isolated CAKUT, the most common cause of childhood CKD. However, these genes account for only 20% of cases. ARHGEF6, a guanine nucleotide exchange factor that is implicated in biologic processes such as cell migration and focal adhesion, acts downstream of integrin-linked kinase (ILK) and parvin proteins. A genetic variant of ILK that causes murine renal agenesis abrogates the interaction of ILK with a murine focal adhesion protein encoded by Parva , leading to CAKUT in mice with this variant.
Methods: To identify novel genes that, when mutated, result in CAKUT, we performed exome sequencing in an international cohort of 1265 families with CAKUT. We also assessed the effects in vitro of wild-type and mutant ARHGEF6 proteins, and the effects of Arhgef6 deficiency in mouse and frog models.
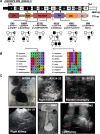
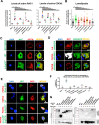
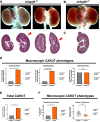
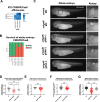
Results: We detected six different hemizygous variants in the gene ARHGEF6 (which is located on the X chromosome in humans) in eight individuals from six families with CAKUT. In kidney cells, overexpression of wild-type ARHGEF6 -but not proband-derived mutant ARHGEF6 -increased active levels of CDC42/RAC1, induced lamellipodia formation, and stimulated PARVA-dependent cell spreading. ARHGEF6-mutant proteins showed loss of interaction with PARVA. Three-dimensional Madin-Darby canine kidney cell cultures expressing ARHGEF6-mutant proteins exhibited reduced lumen formation and polarity defects. Arhgef6 deficiency in mouse and frog models recapitulated features of human CAKUT.
Conclusions: Deleterious variants in ARHGEF6 may cause dysregulation of integrin-parvin-RAC1/CDC42 signaling, thereby leading to X-linked CAKUT.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
F. Hildebrandt is a cofounder and S.A.B. member of Goldfinch Biopharma Inc. F. Hildebrandt also reports Research Funding: NIH; Honoraria: Sanofi; Patents or Royalties: NPHP1; and Advisory or Leadership Role: Goldfinch-Bio. K.M. Kirschner reports Ownership Interest: Bio-Techne. E. Banne reports Consultancy: Pronto Diagnostics, Israel; and Research Funding: Rhythm Pharmaceuticals. R.P. Lifton reports Advisory or Leadership Role: Roche Board of Directors, and Genentech Board of Directors. A.J. Majmundar reports Consultancy: Judo Bio, Inc. C.-H.W. Wu reports Advisory or Leadership Role: GENE (journal), and The Association of Chinese Geneticists in America (ACGA). All remaining authors have nothing to disclose.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

