Targeting stressor-induced dysfunctions in protein-protein interaction networks via epichaperomes
- PMID: 36414432
- PMCID: PMC9789192
- DOI: 10.1016/j.tips.2022.10.006
Targeting stressor-induced dysfunctions in protein-protein interaction networks via epichaperomes
Abstract
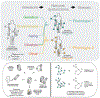
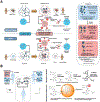
Diseases are manifestations of complex changes in protein-protein interaction (PPI) networks whereby stressors, genetic, environmental, and combinations thereof, alter molecular interactions and perturb the individual from the level of cells and tissues to the entire organism. Targeting stressor-induced dysfunctions in PPI networks has therefore become a promising but technically challenging frontier in therapeutics discovery. This opinion provides a new framework based upon disrupting epichaperomes - pathological entities that enable dysfunctional rewiring of PPI networks - as a mechanism to revert context-specific PPI network dysfunction to a normative state. We speculate on the implications of recent research in this area for a precision medicine approach to detecting and treating complex diseases, including cancer and neurodegenerative disorders.
Keywords: Alzheimer's disease; cancer; epichaperome; network medicine; neurodegenerative disorders; protein network dysfunction; protein–protein interaction networks.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MSKCC holds the intellectual rights to the epichaperome portfolio. G.C. and L.N. share partial ownership and are members of the board of directors at Samus Therapeutics Inc, which has licensed this portfolio. G.C. and S.S. are inventors on the licensed intellectual property. Other disclosures for L.N. are listed at https://www.mskcc.org/cancer-care/doctors/larry-norton. S.D.G. declares no competing interests.
Figures
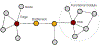

References
-
- Greene JA and Loscalzo J (2017) Putting the Patient Back Together - Social Medicine, Network Medicine, and the Limits of Reductionism. N. Engl. J. Med 377, 2493–2499. - PubMed
Publication types
MeSH terms
Grants and funding
- R56 AG061869/AG/NIA NIH HHS/United States
- R21 CA158609/CA/NCI NIH HHS/United States
- RF1 AG061566/AG/NIA NIH HHS/United States
- R56 AG072599/AG/NIA NIH HHS/United States
- RF1 AG071805/AG/NIA NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
- R01 AG074004/AG/NIA NIH HHS/United States
- U01 AG032969/AG/NIA NIH HHS/United States
- R01 AG085572/AG/NIA NIH HHS/United States
- R01 AG067598/AG/NIA NIH HHS/United States
- R21 AI090501/AI/NIAID NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- P01 CA186866/CA/NCI NIH HHS/United States
- P01 AG017617/AG/NIA NIH HHS/United States
- R01 CA172546/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

