COVID-19 vaccine acceptance and its socio-demographic and emotional determinants: A multi-country cross-sectional study
- PMID: 36414475
- PMCID: PMC9647027
- DOI: 10.1016/j.vaccine.2022.10.051
COVID-19 vaccine acceptance and its socio-demographic and emotional determinants: A multi-country cross-sectional study
Abstract
Background: Multiple COVID-19 vaccines have now been licensed for human use, with other candidate vaccines in different stages of development. Effective and safe vaccines against COVID-19 have been essential in achieving global reductions in severe disease caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), but multiple factors, including vaccine supply and vaccine confidence, continue to impact global uptake of COVID-19 vaccines. In this study, we explore determinants of COVID-19 vaccination intent across17 countries worldwide.
Methods: In this large-scale multi-country study, we explored intent to accept a COVID-19 vaccine and the socio-demographic and emotional determinants of uptake for 17 countries and over 19,000 individuals surveyed in June and July 2020 via nationally representative samples. We used Bayesian ordinal logistic regressions to probe the relationship between intent to accept a COVID-19 vaccine and individuals' socio-demographic status, their confidence in COVID-19 vaccines, and their recent emotional status. Gibbs sampling was used for Bayesian model inference, with 95% Bayesian highest posterior density intervals used to capture uncertainty.
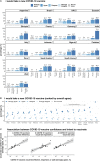
Findings: Intent to accept a COVID-19 vaccine was found to be highest in India, where 77⋅8% (95% HPD, 75⋅5 to 80⋅0%) of respondents strongly agreeing that they would take a new COVID-19 vaccine if it were available. The Democratic Republic of Congo (15⋅5%, 12⋅2 to 18⋅6%) and France (26⋅4%, 23⋅7 to 29⋅2%) had the lowest share of respondents who strongly agreed that they would accept a COVID-19. Confidence in the safety, importance, and effectiveness of COVID-19 vaccines are the most widely informative determinants of vaccination intent. Socio-demographic and emotional determinants played a lesser role, with being male and having higher education associated with increased uptake intent in five countries and being fearful of catching COVID-19 also a strong determinant of uptake intent.
Interpretation: Barriers to COVID-19 vaccine acceptance are found to be country and context dependent. These findings highlight the importance of regular monitoring of COVID-19 vaccine confidence to identify groups less likely to vaccinate.
Keywords: COVID-19; SARS-CoV-2; Vaccine confidence; Vaccine hesitancy.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: [AdF, CS and HJL are involved in collaborative grants with GlaxoSmithKline, Merck and Johnson & Johnson. HJL has also received other support for participating in Merck meetings and GlaxoSmithKline advisory roundtables; HJL is a member of the Merck Vaccine Confidence Advisory Board].
Figures




References
-
- Bernal, J.L., Andrews, N., Gower, C., Stowe, J., Robertson, C., Tessier, E., Simmons, R., Cottrell, S., Robertson, R., O’Doherty, M. and Brown, K. Early effectiveness of COVID-19 vaccination with BNT162b2 mRNA vaccine and ChAdOx1 adenovirus vector vaccine on symptomatic disease, hospitalisations and mortality in older adults in England. medRxiv (not peer-reviewed) (2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

