Manganese efflux transporter SLC30A10 missense polymorphism T95I associated with liver injury retains manganese efflux activity
- PMID: 36414535
- PMCID: PMC9829465
- DOI: 10.1152/ajpgi.00213.2022
Manganese efflux transporter SLC30A10 missense polymorphism T95I associated with liver injury retains manganese efflux activity
Abstract
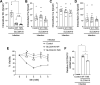
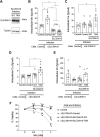
The activity of the manganese (Mn) efflux transporter SLC30A10 in the liver and intestines is critical for Mn excretion and preventing Mn toxicity. Homozygous loss-of-function mutations in SLC30A10 are a well-established cause of hereditary Mn toxicity. But, the relationship between more common SLC30A10 polymorphisms, Mn homeostasis, and disease is only recently emerging. In 2021, the first coding SNP in SLC30A10 (T95I) was associated with liver disease raising the hypothesis that the T95I substitution may induce disease by inhibiting the Mn efflux function of SLC30A10. Here, we test this hypothesis using structural, viability, and metal quantification approaches. Analyses of a predicted structure of SLC30A10 revealed that the side chain of T95 pointed away from the putative Mn-binding cavity, raising doubts about the impact of the T95I substitution on SLC30A10 function. In HeLa or HepG2 cells, overexpression of SLC30A10-WT or T95I resulted in comparable reductions of intracellular Mn levels and protection against Mn-induced cell death. Furthermore, ΔSLC30A10 HepG2 cells, generated using CRISPR/Cas9, exhibited elevated Mn levels and heightened sensitivity to Mn-induced cell death, and these phenotypic changes were similarly rescued by expression of SLC30A10-WT or T95I. Finally, turnover rates of SLC30A10-WT or T95I were also comparable. In summary, our results indicate that the Mn transport activity of SLC30A10-T95I is essentially comparable to the WT protein. Our findings imply that SLC30A10-T95I either has a complex association with liver injury that extends beyond the simple reduction in SLC30A10 activity or alternatively the T95I mutation lacks a causal role in liver disease.NEW & NOTEWORTHY This study demonstrates that the T95I polymorphism in the manganese transporter SLC30A10, which has been associated with liver disease in human GWAS studies, does not impact transporter function in cell culture. These findings raise doubts about the causal relationship of the T95I polymorphism with human disease and highlight the importance of validating GWAS findings using mechanistic approaches.
Keywords: ZnT10; excretion; homeostasis; metal; transporter.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

