Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study
- PMID: 36414626
- PMCID: PMC9681874
- DOI: 10.1038/s41398-022-02254-9
Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study
Abstract
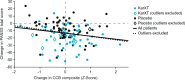
The muscarinic receptor agonist xanomeline improved cognition in phase 2 trials in Alzheimer's disease and schizophrenia. We present data on the effect of KarXT (xanomeline-trospium) on cognition in schizophrenia from the 5-week, randomised, double-blind, placebo-controlled EMERGENT-1 trial (NCT03697252). Analyses included 125 patients with computerised Cogstate Brief Battery (CBB) subtest scores at baseline and endpoint. A post hoc subgroup analysis evaluated the effects of KarXT on cognitive performance in patients with or without clinically meaningful cognitive impairment at baseline, and a separate outlier analysis excluded patients with excessive intraindividual variability (IIV) across cognitive subdomains. ANCOVA models assessed treatment effects for completers and impairment subgroups, with or without removal of outliers. Sample-wide, cognitive improvement was numerically but not statistically greater with KarXT (n = 60) than placebo (n = 65), p = 0.16. However, post hoc analyses showed 65 patients did not exhibit clinically meaningful cognitive impairment at baseline, while eight patients had implausibly high IIV at one or both timepoints. Significant treatment effects were observed after removing outliers (KarXT n = 54, placebo n = 63; p = 0.04). Despite the small sample size, a robust (d = 0.50) and significant effect was observed among patients with cognitive impairment (KarXT n = 23, placebo n = 37; p = 0.03). These effects did not appear to be related to improvement in PANSS total scores (linear regression, R2 = 0.03). Collectively, these findings suggest that KarXT may have a separable and meaningful impact on cognition, particularly among patients with cognitive impairment.
© 2022. The Author(s).
Conflict of interest statement
CS, ACM, SMP, and SKB are employees of and hold equity in Karuna Therapeutics. LAA and EB provide consulting services for Karuna Therapeutics.
Figures
References
-
- Keefe RSE, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;11–37. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical