Angiopoietin-like 2 is essential to aortic valve development in mice
- PMID: 36414704
- PMCID: PMC9681843
- DOI: 10.1038/s42003-022-04243-6
Angiopoietin-like 2 is essential to aortic valve development in mice
Abstract
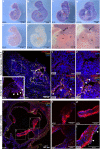
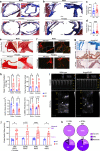
Aortic valve (AoV) abnormalities during embryogenesis are a major risk for the development of aortic valve stenosis (AVS) and cardiac events later in life. Here, we identify an unexpected role for Angiopoietin-like 2 (ANGPTL2), a pro-inflammatory protein secreted by senescent cells, in valvulogenesis. At late embryonic stage, mice knocked-down for Angptl2 (Angptl2-KD) exhibit a premature thickening of AoV leaflets associated with a dysregulation of the fine balance between cell apoptosis, senescence and proliferation during AoV remodeling and a decrease in the crucial Notch signalling. These structural and molecular abnormalities lead toward spontaneous AVS with elevated trans-aortic gradient in adult mice of both sexes. Consistently, ANGPTL2 expression is detected in human fetal semilunar valves and associated with pathways involved in cell cycle and senescence. Altogether, these findings suggest that Angptl2 is essential for valvulogenesis, and identify Angptl2-KD mice as an animal model to study spontaneous AVS, a disease with unmet medical need.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

