Brain sex-dependent alterations after prolonged high fat diet exposure in mice
- PMID: 36414721
- PMCID: PMC9681749
- DOI: 10.1038/s42003-022-04214-x
Brain sex-dependent alterations after prolonged high fat diet exposure in mice
Abstract
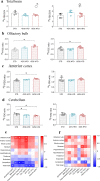
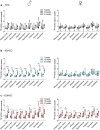
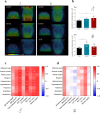
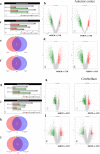
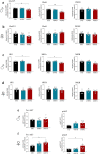
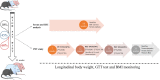
We examined effects of exposing female and male mice for 33 weeks to 45% or 60% high fat diet (HFD). Males fed with either diet were more vulnerable than females, displaying higher and faster increase in body weight and more elevated cholesterol and liver enzymes levels. Higher glucose metabolism was revealed by PET in the olfactory bulbs of both sexes. However, males also displayed altered anterior cortex and cerebellum metabolism, accompanied by a more prominent brain inflammation relative to females. Although both sexes displayed reduced transcripts of neuronal and synaptic genes in anterior cortex, only males had decreased protein levels of AMPA and NMDA receptors. Oppositely, to anterior cortex, cerebellum of HFD-exposed mice displayed hypometabolism and transcriptional up-regulation of neuronal and synaptic genes. These results indicate that male brain is more susceptible to metabolic changes induced by HFD and that the anterior cortex versus cerebellum display inverse susceptibility to HFD.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

