Comparative Effectiveness of Device-Aided Therapies on Quality of Life and Off-Time in Advanced Parkinson's Disease: A Systematic Review and Bayesian Network Meta-analysis
- PMID: 36414908
- PMCID: PMC9712309
- DOI: 10.1007/s40263-022-00963-9
Comparative Effectiveness of Device-Aided Therapies on Quality of Life and Off-Time in Advanced Parkinson's Disease: A Systematic Review and Bayesian Network Meta-analysis
Abstract
Introduction: Research comparing levodopa/carbidopa intestinal gel (LCIG), deep brain stimulation (DBS), and continuous subcutaneous apomorphine infusion (CSAI) for advanced Parkinson's disease (PD) is lacking. This network meta-analysis (NMA) assessed the comparative effectiveness of LCIG, DBS, CSAI and best medical therapy (BMT) in reducing off-time and improving quality of life (QoL) in patients with advanced PD.
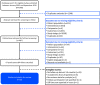
Methods: A systematic literature review was conducted for randomized controlled trials (RCTs), observational and interventional studies from January 2003 to September 2019. Data extracted at baseline and 6 months were off-time, as reported by diary or Unified Parkinson's Disease Rating Scale Part IV item 39, and QoL, as reported by Parkinson's Disease Questionnaire (PDQ-39/PDQ-8). Bayesian NMA was performed to estimate pooled treatment effect sizes and to rank treatments in order of effectiveness.
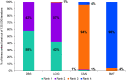
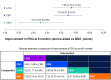
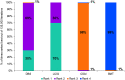
Results: A total of 22 studies fulfilled the inclusion criteria (n = 2063 patients): four RCTs, and 16 single-armed, one 2-armed and one 3-armed prospective studies. Baseline mean age was between 55.5-70.9 years, duration of PD was 9.1-15.3 years, off-time ranged from 5.4 to 8.7 h/day in 9 studies, and PDQ scores ranged from 28.8 to 67.0 in 19 studies. Levodopa/carbidopa intestinal gel and DBS demonstrated significantly greater improvement in off-time and QoL at 6 months compared with CSAI and BMT (p < 0.05). There was no significant difference in the effects of LCIG and DBS, but DBS was ranked first for reduction in off-time, and LCIG was ranked first for improvement in QoL.
Conclusions: This NMA found that LCIG and DBS were associated with superior improvement in off-time and PD-related QoL compared with CSAI and BMT at 6 months after treatment initiation. This comparative effectiveness research may assist providers, patients, and caregivers in the selection of the optimal device-aided therapy.
© 2022. The Author(s).
Conflict of interest statement
Angelo Antonini has received compensation for consultancy and speaker-related activities from UCB, Boehringer Ingelheim, Britannia, AbbVie, Kyowa Kirin, Zambon, Bial, Neuroderm, Theravance Biopharma, Roche. He receives research support from Chiesi Pharmaceuticals, Lundbeck, Bial, Horizon2020 Grant 825785, Horizon2020 Grant 101016902, Ministry of Education University and Research (MIUR) Grant ARS01_01081, Cariparo Foundation. He serves as consultant for Boehringer–Ingelheim for legal cases on pathological gambling. Rajesh Pahwa has received consulting fees from AbbVie, ACADIA, Acorda, Adamas, Cynapsus, Global Kinetics, Lundbeck, Neurocrine, Pfizer, Sage, Sunovion, Teva Neuroscience and US World Meds. He has received research grants from AbbVie, Adamas, Avid, Biotie, Boston Scientific, Civitas, Cynapsus, Kyowa, National Parkinson Foundation, NIH/NINDS, Parkinson Study Group. Per Odin has received compensations for consultancy and speaker-related activities from AbbVie, Bial, Britannia, Ever Pharma, Lobsor, Nordic Infucare, Stada, and Zambon. He has received royalties from Uni-Med Verlag. Stuart Isaacson has received honoraria for CME, consultant, research grants, and/or promotional speaker on behalf of AbbVie, Acadia, Acorda, Adamas, Addex, Affiris, Alexva, Allergan, Amarantus, Amneal, Aptinyx, Axial, Axovant, Benevolent, Biogen, Britannia, Cadent, Cala, Cerecor, Cerevel, Cipla, Eli Lilly, Enterin, GE Healthcare, Global Kinetics, Impax, Impel, Intec Pharma, Ipsen, Jazz, Kyowa, Lundbeck, Merz, Michael J. Fox Foundation, Mitsubishi Tanabe, Neuralys, Neurocrine, Neuroderm, Parkinson Study Group, Pharma2B, Prilenia, Promentis, Revance, Roche, Sanofi, Sunovion, Sun Pharma, Supernus, Teva, Theravance, UCB. Aristide Merola has received grant support from the NIH (KL2 TR001426). He has received speaker honoraria or advisory board honoraria from CSL Behring, AbbVie, Abbott, Medtronic, Theravance, and Cynapsus Therapeutics. He has received grant support from Lundbeck and AbbVie. Lin Wang, Lakshmi P. Kandukuri, Ali Alobaidi, Yanjun Bao, Cindy Zadikoff, Juan Carlos Parra, Lars Bergmann, and Connie H. Yan are employees of AbbVie and may own stocks/shares in the company. K. Ray Chaudhuri has received educational funding from UCB, and honoraria for sponsored symposia from UCB, AbbVie, Britannia, US Worldmeds, Otsuka, Medtronic, Zambon and acted as a consultant for AbbVie, UCB, Britannia, Medtronic, and Mundipharma.
Figures

Similar articles
-
The Long-Term Impact of Levodopa/Carbidopa Intestinal Gel on 'Off'-time in Patients with Advanced Parkinson's Disease: A Systematic Review.Adv Ther. 2021 Jun;38(6):2854-2890. doi: 10.1007/s12325-021-01747-1. Epub 2021 May 20. Adv Ther. 2021. PMID: 34018146 Free PMC article.
-
Levodopa-Carbidopa Intestinal Gel in Patients with Parkinson's Disease: A Systematic Review.CNS Drugs. 2016 May;30(5):381-404. doi: 10.1007/s40263-016-0336-5. CNS Drugs. 2016. PMID: 27138916
-
Continuous, subcutaneous apomorphine infusion for Parkinson disease motor fluctuations: Results from the phase 3, long-term, open-label United States InfusON study.J Parkinsons Dis. 2025 Mar;15(2):361-373. doi: 10.1177/1877718X241310727. Epub 2025 Jan 29. J Parkinsons Dis. 2025. PMID: 39973510 Clinical Trial.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Satisfaction and Preferences for Infusion Therapies in Advanced Parkinson's Disease-Patient Perspective.Medicina (Kaunas). 2024 Dec 28;61(1):27. doi: 10.3390/medicina61010027. Medicina (Kaunas). 2024. PMID: 39859009 Free PMC article.
-
Levodopa-carbidopa intestinal gel in advanced Parkinson's disease: long-term results from COSMOS.J Neurol. 2023 May;270(5):2765-2775. doi: 10.1007/s00415-023-11615-3. Epub 2023 Feb 18. J Neurol. 2023. PMID: 36802031 Free PMC article.
-
Tools and criteria to select patients with advanced Parkinson's disease for device-aided therapies: a narrative review.J Neural Transm (Vienna). 2023 Nov;130(11):1359-1377. doi: 10.1007/s00702-023-02656-z. Epub 2023 Jul 27. J Neural Transm (Vienna). 2023. PMID: 37500937 Free PMC article. Review.
-
Quality of Life in Patients With Parkinson's Disease: A Cross-Sectional Study.Cureus. 2023 Jan 20;15(1):e33989. doi: 10.7759/cureus.33989. eCollection 2023 Jan. Cureus. 2023. PMID: 36824559 Free PMC article.
-
Therapeutic innovations for the symptomatic treatment of Parkinson's disease: focus on technology-based therapies.J Neural Transm (Vienna). 2025 Mar 22. doi: 10.1007/s00702-025-02915-1. Online ahead of print. J Neural Transm (Vienna). 2025. PMID: 40119890 Review.
References
-
- Antonini A, Stoessl AJ, Kleinman LS, Skalicky AM, Marshall TS, Sail KR, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson's disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018;34(12):2063–2073. doi: 10.1080/03007995.2018.1502165. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

