Defb19 regulates the migration of germ cell and is involved in male fertility
- PMID: 36414976
- PMCID: PMC9682749
- DOI: 10.1186/s13578-022-00924-1
Defb19 regulates the migration of germ cell and is involved in male fertility
Abstract
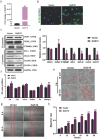
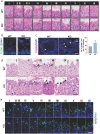
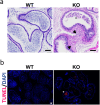
β-defensins are small antimicrobial peptides that play essential roles in male fertility. Although several members of the β-defensin family are preferentially expressed in the testis, their involvement in spermatogenesis remains elusive. In this study, we have characterized the expression and function of Defb19 in mouse testis. Our results showed that Defb19 is expressed in both Sertoli cells and germ cells. Overexpression of Defb19 in the 15P-1 Sertoli cell line decreases the expression of cell junction molecules and promotes the matrix adhesion and migration of Sertoli cells. Recombinant DEFB19 and conditioned medium of Defb19-overexpressed 15P-1 cells promote the migration of GC2-spd spermatocyte cell line. Knockout of Defb19 in mouse by CRISPR/Cas9 resulted in male subfertility with testicular and epididymal atrophy. A marked increase in apoptosis and a significant decrease in the sperm count were observed in the KO mice. Together, our study has uncovered an important role of Defb19 in male fertility by regulating the migration of both the Sertoli cells and the germ cells. Our study has shed new light on the functions of β-defensins in the testis.
Keywords: Cell migration; Male fertility; Spermatogenesis; β-defensin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice.Endocr Rev. 2009 Apr;30(2):119-32. doi: 10.1210/er.2008-0025. Epub 2009 Jan 27. Endocr Rev. 2009. PMID: 19176467 Free PMC article. Review.
-
β-Defensin 19/119 mediates sperm chemotaxis and is associated with idiopathic infertility.Cell Rep Med. 2022 Dec 20;3(12):100825. doi: 10.1016/j.xcrm.2022.100825. Epub 2022 Dec 12. Cell Rep Med. 2022. PMID: 36513070 Free PMC article.
-
Specific deletion of Cdh2 in Sertoli cells leads to altered meiotic progression and subfertility of mice.Biol Reprod. 2015 Mar;92(3):79. doi: 10.1095/biolreprod.114.126334. Epub 2015 Jan 28. Biol Reprod. 2015. PMID: 25631347
-
Antimicrobial protection of the mouse testis: synthesis of defensins of the cryptdin family.Biol Reprod. 1997 Nov;57(5):1115-22. doi: 10.1095/biolreprod57.5.1115. Biol Reprod. 1997. PMID: 9369178
-
Roles of connexins in testis development and spermatogenesis.Semin Cell Dev Biol. 2016 Feb;50:22-30. doi: 10.1016/j.semcdb.2015.12.019. Epub 2016 Jan 11. Semin Cell Dev Biol. 2016. PMID: 26780117 Review.
Cited by
-
DEFB119 stratifies dysbiosis with distorted networks in the seminal microbiome associated with male infertility.PNAS Nexus. 2024 Sep 19;3(10):pgae419. doi: 10.1093/pnasnexus/pgae419. eCollection 2024 Oct. PNAS Nexus. 2024. PMID: 39359400 Free PMC article.
-
Guilu erxian glue improves sperm quality in oligasthenospermia mice by promoting TDRP methylation and regulating the cAMP/PKA pathway.Sci Rep. 2025 Jul 28;15(1):27498. doi: 10.1038/s41598-025-09667-8. Sci Rep. 2025. PMID: 40721586 Free PMC article.
-
β-Defensin gene copy number variation in cattle.R Soc Open Sci. 2024 Oct 30;11(10):241154. doi: 10.1098/rsos.241154. eCollection 2024 Oct. R Soc Open Sci. 2024. PMID: 39479249 Free PMC article.
-
A Single-Cell Landscape of Spermioteleosis in Mice and Pigs.Cells. 2024 Mar 22;13(7):563. doi: 10.3390/cells13070563. Cells. 2024. PMID: 38607002 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

