Usefulness of endocytoscopy in evaluating transbronchial biopsy specimens
- PMID: 36415054
- PMCID: PMC9834692
- DOI: 10.1111/1759-7714.14731
Usefulness of endocytoscopy in evaluating transbronchial biopsy specimens
Abstract
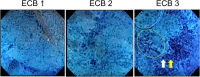
Background: Endocytoscopy (ECS) provides a magnification of approximately 450× for real-time observation of lesion nuclei. Using ECS, we aimed to evaluate whether sufficient samples for diagnosis can be obtained during bronchoscopy. We also investigated whether ECS can enable two-class diagnosis of malignant or non-malignant transbronchial biopsy specimens in real-time during bronchoscopy.
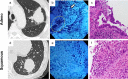
Methods: This was a single-facility, prospective, observational, ex vivo study. Forty cases with localized peripheral pulmonary lesions underwent transbronchial biopsy with endobronchial ultrasonography using a guide sheath. Each biopsy specimen was immediately observed and evaluated endocytoscopically after the collection by the bronchoscopic procedure.
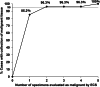
Results: Thirty-seven cases were enrolled. The diagnostic accuracy achieved by ECS was 91.9% (34/37). The agreement rate between the endocytoscopic evaluation and pathological diagnosis of each specimen (170 specimens) was 65.3% (111/170). The median time required for endocytoscopic evaluation per specimen was 70 s. When we judged a specimen to be malignant a second time on ECS evaluations of five specimens in one case, pathologically malignant specimens were collected in 26 of 27 cases (96.3%).
Conclusions: ECS with methylene blue staining may aid in the two-class diagnosis of malignant or non-malignant transbronchial biopsy specimens during bronchoscopy. This may reduce the number of tissue biopsies.
Keywords: biopsybronchoscopyendocytoscopy.
© 2022 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Takae Okuno has no conflicts of interest. Noriaki Kurimoto received personal fees from Olympus Corporation. Noriaki Kurimoto received personal fees from Eli Lilly Japan K.K., and Chugai Pharmaceutical outside the submitted work. Akari Tanino has no conflicts of interest. Megumi Hamaguchi has no conflicts of interest. Takamasa Hotta has no conflicts of interest. Ryosuke Tanino has no conflicts of interest. Misato Kobayashi has no conflicts of interest. Yohei Shiratsuki has no conflicts of interest. Shunichi Hamaguchi has no conflicts of interest. Takeshi Isobe received grants from KONICA MINOLTA, IQVIA Services JAPAN K.K. and Insmed outside the submitted work. Takeshi Isobe received personal fees from DAIICHI SANKYO COMPANY, AstraZeneca K.K., and Nippon Boehringer Ingelheim outside the submitted work. Yukari Tsubata received grants from ONO PHARMACEUTICAL and Pfizer Health Research Foundation., outside the submitted work. Yukari Tsubata received personal fees from DAIICHI SANKYO COMPANY, AstraZeneca K.K., and Chugai Pharmaceutical, outside the submitted work.
Figures



References
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. - PubMed
-
- Kumagai Y, Kawada K, Higashi M, et al. Endocytoscopic observation of various esophageal lesions at ×600: can nuclear abnormality be recognized? Dis Esophagus. 2005;28:269–75. - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical

