Four SARS-CoV-2 vaccine doses or hybrid immunity in patients on immunosuppressive therapies: a Norwegian cohort study
- PMID: 36415604
- PMCID: PMC9671616
- DOI: 10.1016/S2665-9913(22)00330-7
Four SARS-CoV-2 vaccine doses or hybrid immunity in patients on immunosuppressive therapies: a Norwegian cohort study
Abstract
Background: Data on response and safety of repeated vaccinations and hybrid immunity in patients with immune-mediated inflammatory diseases on immunosuppressive therapy is needed to further develop vaccination strategies in this vulnerable population. This study aimed to evaluate hybrid immunity and humoral immune response and safety of four SARS-CoV-2 vaccine doses in patients with immune-mediated inflammatory diseases on immunosuppressive therapy.
Methods: This prospective observational Norwegian study of vaccine response to COVID-19 (Nor-vaC) included adult patients aged 18 years and older with immune-mediated inflammatory diseases (rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, Crohn's disease, or ulcerative colitis) on immunosuppressive therapy, who had received four SARS-CoV-2 vaccine doses (vaccine group) or three vaccine doses followed by COVID-19 (hybrid group), and healthy controls receiving three vaccine doses (control group). Patients were recruited from the Division of Rheumatology at Diakonhjemmet Hospital, Oslo, and the Department of Gastroenterology at Akershus University Hospital, Lørenskog. Patients who had COVID-19 before the third vaccine dose, and patients with allergies or intolerances to elements of the vaccine were excluded. Antibodies to the receptor-binding domain of SARS-CoV-2 spike protein (anti-RBD antibodies) were assessed 2-4 weeks following vaccination or COVID-19. This study is registered at Clinialtrials.gov, NCT04798625.
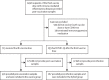
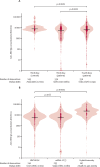
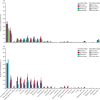
Findings: Between Nov 12, 2021, and April 19, 2022, 1458 participants with immune-mediated inflammatory diseases provided post-vaccination samples at 2-4 weeks following a third vaccine dose. After 544 participants were excluded, 715 (78%) of the remaining 914 participants received the fourth dose of the vaccine, and of these, 536 (75%) provided post-vaccination samples 2-4 weeks after their fourth vaccination (vaccine group). 199 (22%) of the 914 had COVID-19 after their third dose of the vaccine and of these, 167 (84%) provided samples (hybrid group). 256 of the eligible 703 patients had rheumatoid arthritis, 107 had spondyloarthritis, 115 had psoriatic arthritis, 130 had Crohn's disease, and 95 had ulcerative colitis). Median age was 56 years [IQR 45-65], 398 (57%) were women, and 305 (43%) were men. Patients in the vaccine group had higher anti-RBD antibody concentrations following the fourth vaccine dose (median 6192 BAU/ml [IQR 2878-11 243]) than after the third dose (median 5087 BAU/ml [1250-9081]; p< 0·0001), but lower antibody concentrations than the control group following the third dose (median 7595 BAU/ml [5916-12 001]; p< 0·0001). Antibody concentrations were higher in the patients in the hybrid group (23 548 BAU/ml [IQR 11 440-35 935]) than in the vaccine group (p<0·0001). No difference was found in antibody concentrations between the fourth dose of BNT162b2 (full-dose) and mRNA-1273 (half-dose). Patients and controls had a comparable safety profile after both three and four vaccine doses.
Interpretation: Vaccine boosters improve humoral immune responses and are safe in patients with immune-mediated inflammatory diseases on immunosuppressive therapy, and administration should be considered regularly in this patient group. Hybrid immunity with omicron induces a strong humoral response suggesting longer intervals between booster doses in this patient group.
Funding: The South-Eastern Norway Regional Health Authority, The Coalition for Epidemic Preparedness Innovations, Akershus University Hospital.
© 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
KHB reports funding from Akershus University Hospital and speaker bureaus for Janssen-Cilag. TKK reports grants from AbbVie, Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Union Chimique Belge; consulting fees from AbbVie, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz, Sanofi; and speakers bureaus from Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz, and Sanofi. JJ reports grants from Boehringer-Ingelheim; speakers bureaus from AbbVie, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz, and Takeda; and participation on advisory board for AbbVie, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz, and Takeda. LAM reports funding from KG Jebsen foundation, support for infrastructure and biobanking from the university of Oslo and Oslo University Hospital, grants from the Coalition of Epidemic Preparedness Innovations, and speakers bureaus from Novartis, and Cellgene. EAH reports consultant fees from AbbVie, Boehringer-Ingelheim, Eli Lilly, and Gilead; and speakers bureaus from Pfizer and UCB. GG reports speaker bureaus from Bayer, Sanofi, ThermoFisher, and participation on advisory board for AstraZeneca. JTV reports grants from the Coalition of Epidemic Preparedness Innovations. GLG reports funding from the South-Eastern Norway Regional Health Authority, The Coalition for Epidemic Preparedness Innovations, RCN Covid (312693), a KG Jebsen Foundation (grant 19), Dr Trygve Gythfeldt og frues forskningsfond, Karin Fossum Foundation, the Research Foundation at Diakonhjemmet Hospital, and Oslo University Hospital; speakers bureaus from AbbVie, Galapagos, Pfizer, and Union Chimique Belge; and participation on advisory board of AbbVie, Galapagos, Pfizer, and Union Chimique Belge. KKJ reports speakers bureaus from Bristol-Myers Squibb and Roche. All other authors declare no competing interests.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

