Metal-binding proteins and cross-linking in the defensive glue of the slug Arion subfuscus
- PMID: 36415975
- PMCID: PMC9682298
- DOI: 10.1098/rsif.2022.0611
Metal-binding proteins and cross-linking in the defensive glue of the slug Arion subfuscus
Abstract
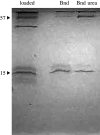
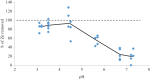
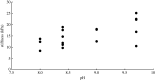
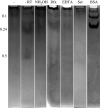
The role of metals in forming the primary cross-links in slug glue was investigated. Several metal-binding proteins were identified in the defensive glue produced by the slug Arion subfuscus. Notably, the C-lectins that are unique to the glue are iron-binding proteins. This is unusual for C-lectins. Dissociating these proteins from iron does not affect the glue's stiffness. Similarly, several proteins that can bind to zinc were identified, but dissociating the proteins from zinc did not weaken the glue. These results suggest that metal coordination is not involved in the primary cross-links of this hydrogel glue. The stable cross-links that provide stiffness are more likely to be created by a catalytic event involving protein oxidation. Cross-linking was unexpectedly difficult to prevent. Collecting the glue into a large volume of ice-cold buffer with reagents aimed at inhibiting oxidative cross-linking caused a slight loss of cross-linking, as demonstrated by the appearance of uncross-linked proteins in native gel electrophoresis. Notable among these was a protein that is normally heavily oxidized (asmp165). Nevertheless, this effect was not large, suggesting that the primary cross-links form before secretion.
Keywords: C-lectin; adhesive; hydrogel; iron; protein oxidation; transition metals.
Figures


Similar articles
-
The relative contribution of calcium, zinc and oxidation-based cross-links to the stiffness of Arion subfuscus glue.J Exp Biol. 2013 Apr 15;216(Pt 8):1475-83. doi: 10.1242/jeb.077149. Epub 2012 Dec 21. J Exp Biol. 2013. PMID: 23264483
-
Elasticity and energy dissipation in the double network hydrogel adhesive of the slug Arion subfuscus.Philos Trans R Soc Lond B Biol Sci. 2019 Oct 28;374(1784):20190201. doi: 10.1098/rstb.2019.0201. Epub 2019 Sep 9. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31495311 Free PMC article.
-
Strong, Non-specific Adhesion Using C-Lectin Heterotrimers in a Molluscan Defensive Secretion.Integr Comp Biol. 2021 Oct 14;61(4):1440-1449. doi: 10.1093/icb/icab100. Integr Comp Biol. 2021. PMID: 34048555
-
Analysis of the adhesive secreting cells of Arion subfuscus: insights into the role of microgels in a tough, fast-setting hydrogel glue.Soft Matter. 2024 Jun 20;20(24):4669-4680. doi: 10.1039/d4sm00071d. Soft Matter. 2024. PMID: 38563822
-
Robust cross-links in molluscan adhesive gels: testing for contributions from hydrophobic and electrostatic interactions.Comp Biochem Physiol B Biochem Mol Biol. 2009 Feb;152(2):110-7. doi: 10.1016/j.cbpb.2008.10.004. Epub 2008 Oct 15. Comp Biochem Physiol B Biochem Mol Biol. 2009. PMID: 18952190 Free PMC article.
Cited by
-
De novo genome assembly and transcriptome sequencing in foot and mantle tissues of Megaustenia siamensis reveals components of adhesive substances.Sci Rep. 2024 Jun 14;14(1):13756. doi: 10.1038/s41598-024-64425-6. Sci Rep. 2024. PMID: 38877053 Free PMC article.
References
-
- Smith AM. 2016. The biochemistry and mechanics of gastropod adhesive gels. In Biological adhesives (ed. Smith AM), pp. 177-192. Cham, Switzerland: Springer.
-
- Denny MW. 1983. Molecular biomechanics of molluscan mucous secretions. In The Mollusca, vol. I (eds Wilbur K, Simkiss K, Hochachka PW), pp. 431-465. New York, NY: Academic Press.
-
- Smith AM. 2013. Multiple metal-based cross-links: protein oxidation and metal coordination in a biological glue. In Biological and biomimetic adhesives: challenges and opportunities (eds Santos R, Aldred N, Gorb S, Flammang P), pp. 3-15. Cambridge, UK: Royal Society of Chemistry.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

