Macrophage-Derived 25-Hydroxycholesterol Promotes Vascular Inflammation, Atherogenesis, and Lesion Remodeling
- PMID: 36416142
- PMCID: PMC9892282
- DOI: 10.1161/CIRCULATIONAHA.122.059062
Macrophage-Derived 25-Hydroxycholesterol Promotes Vascular Inflammation, Atherogenesis, and Lesion Remodeling
Abstract
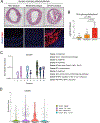
Background: Cross-talk between sterol metabolism and inflammatory pathways has been demonstrated to significantly affect the development of atherosclerosis. Cholesterol biosynthetic intermediates and derivatives are increasingly recognized as key immune regulators of macrophages in response to innate immune activation and lipid overloading. 25-Hydroxycholesterol (25-HC) is produced as an oxidation product of cholesterol by the enzyme cholesterol 25-hydroxylase (CH25H) and belongs to a family of bioactive cholesterol derivatives produced by cells in response to fluctuating cholesterol levels and immune activation. Despite the major role of 25-HC as a mediator of innate and adaptive immune responses, its contribution during the progression of atherosclerosis remains unclear.
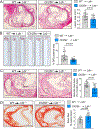
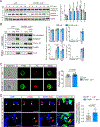
Methods: The levels of 25-HC were analyzed by liquid chromatography-mass spectrometry, and the expression of CH25H in different macrophage populations of human or mouse atherosclerotic plaques, respectively. The effect of CH25H on atherosclerosis progression was analyzed by bone marrow adoptive transfer of cells from wild-type or Ch25h-/- mice to lethally irradiated Ldlr-/- mice, followed by a Western diet feeding for 12 weeks. Lipidomic, transcriptomic analysis and effects on macrophage function and signaling were analyzed in vitro from lipid-loaded macrophage isolated from Ldlr-/- or Ch25h-/-;Ldlr-/- mice. The contribution of secreted 25-HC to fibrous cap formation was analyzed using a smooth muscle cell lineage-tracing mouse model, Myh11ERT2CREmT/mG;Ldlr-/-, adoptively transferred with wild-type or Ch25h-/- mice bone marrow followed by 12 weeks of Western diet feeding.
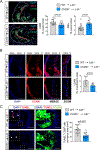
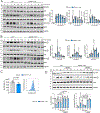
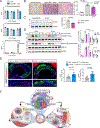
Results: We found that 25-HC accumulated in human coronary atherosclerotic lesions and that macrophage-derived 25-HC accelerated atherosclerosis progression, promoting plaque instability through autocrine and paracrine actions. 25-HC amplified the inflammatory response of lipid-loaded macrophages and inhibited the migration of smooth muscle cells within the plaque. 25-HC intensified inflammatory responses of lipid-laden macrophages by modifying the pool of accessible cholesterol in the plasma membrane, which altered Toll-like receptor 4 signaling, promoted nuclear factor-κB-mediated proinflammatory gene expression, and increased apoptosis susceptibility. These effects were independent of 25-HC-mediated modulation of liver X receptor or SREBP (sterol regulatory element-binding protein) transcriptional activity.
Conclusions: Production of 25-HC by activated macrophages amplifies their inflammatory phenotype, thus promoting atherogenesis.
Keywords: atherosclerosis; inflammation; macrophages; oxysterol.
Conflict of interest statement
Disclosures
None
Figures


References
-
- Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, Gerrity R, Schwartz CJ, Fogelman AM. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990;87:5134–5138. doi: 10.1073/pnas.87.13.5134 - DOI - PMC - PubMed
-
- Hurt-Camejo E, Camejo G, Rosengren B, Lopez F, Ahlstrom C, Fager G, Bondjers G. Effect of arterial proteoglycans and glycosaminoglycans on low density lipoprotein oxidation and its uptake by human macrophages and arterial smooth muscle cells. Arterioscler Thromb. 1992;12:569–583. doi: 10.1161/01.atv.12.5.569 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

