Carbon-based glyco-nanoplatforms: towards the next generation of glycan-based multivalent probes
- PMID: 36416290
- PMCID: PMC9743786
- DOI: 10.1039/d2cs00741j
Carbon-based glyco-nanoplatforms: towards the next generation of glycan-based multivalent probes
Abstract
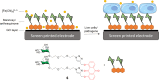
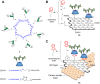
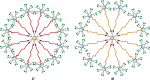
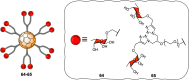
Cell surface carbohydrates mediate a wide range of carbohydrate-protein interactions key to healthy and disease mechanisms. Many of such interactions are multivalent in nature and in order to study these processes at a molecular level, many glycan-presenting platforms have been developed over the years. Among those, carbon nanoforms such as graphene and their derivatives, carbon nanotubes, carbon dots and fullerenes, have become very attractive as biocompatible platforms that can mimic the multivalent presentation of biologically relevant glycosides. The most recent examples of carbon-based nanoplatforms and their applications developed over the last few years to study carbohydrate-mediate interactions in the context of cancer, bacterial and viral infections, among others, are highlighted in this review.
Conflict of interest statement
There are no conflict of interest to report, however MCG wishes to declare that she is co-founder and co-director of CDOTBIO Ltd.
Figures
















References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

