Ethinylestradiol in combined hormonal contraceptive has a broader effect on serum proteome compared with estradiol valerate: a randomized controlled trial
- PMID: 36416543
- PMCID: PMC9825269
- DOI: 10.1093/humrep/deac250
Ethinylestradiol in combined hormonal contraceptive has a broader effect on serum proteome compared with estradiol valerate: a randomized controlled trial
Abstract
Study question: Does an estradiol-based combined oral contraceptive (COC) have a milder effect on the serum proteome than an ethinylestradiol (EE)-based COC or dienogest (DNG) only?
Summary answer: The changes in serum proteome were multifold after the use of a synthetic EE-based COC compared to natural estrogen COC or progestin-only preparation.
What is known already: EE-based COCs widely affect metabolism, inflammation, hepatic protein synthesis and blood coagulation. Studies comparing serum proteomes after the use of COCs containing EE and natural estrogens are lacking.
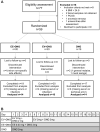
Study design, size, duration: This was a spin-off from a randomized, controlled, two-center clinical trial. Women (n = 59) were randomized to use either EE + DNG, estradiol valerate (EV) + DNG or DNG only continuously for 9 weeks.
Participants/materials, setting, methods: Participants were healthy, young, white volunteer women. Serum samples were collected before and after 9 weeks of hormonal exposure. Samples from 44 women were available for analysis (EE + DNG n = 14, EV + DNG n = 16 and DNG only n = 14). Serum proteins were analyzed by quantitative, discovery-type label-free proteomics.
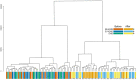
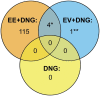
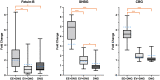
Main results and the role of chance: Altogether, 446 proteins/protein families with two or more unique peptides were detected and quantified. The number of proteins/families that altered over the 9-week period within the study groups was 121 for EE + DNG and 5 for EV + DNG, while no changes were detected for DNG only. When alterations were compared between the groups, significant differences were detected for 63 proteins/protein families, of which 58 were between the EE + DNG and EV + DNG groups. The most affected functions during the use of EE + DNG were the complement system, acute phase response signaling, metabolism and the coagulation system. The results were validated by fetuin-B and cortisol-binding globulin ELISA and sex hormone-binding globulin immunoassay.
Large scale data: Data are available via ProteomeXchange with identifiers PXD033617 (low abundance fraction) and PXD033618 (high abundance fraction).
Limitations, reasons for caution: The power analysis of the trial was not based on the proteomic analysis of this spin-off study. In the future, targeted proteomic analysis with samples from another trial should be carried out in order to confirm the results.
Wider implications of the findings: The EE-based COC exerted a broader effect on the serum proteome than the EV-based COC or the DNG-only preparation. These results demonstrate that the effects of EE in COCs go far beyond the established endpoint markers of estrogen action, while the EV combination is closer to the progestin-only preparation. The study indicates that EV could provide a preferable option to EE in COCs in the future and signals a need for further studies comparing the clinical health outcomes of COCs containing EE and natural estrogens.
Study funding/competing interest(s): Funding for this researcher-initiated study was obtained from the Helsinki University Hospital research funds, the Hospital District of Helsinki and Uusimaa, the Sigrid Juselius Foundation, the Academy of Finland, the Finnish Medical Association, the University of Oulu Graduate School, the Emil Aaltonen Foundation, the Swedish Cultural Foundation in Finland, the Novo Nordisk Foundation, Orion Research Foundation and the Northern Ostrobothnia Regional Fund. The funders had no role in study design, data collection and analysis, publishing decisions or manuscript preparation. T.P. has received honoraria for lectures, consultations and research grants from Exeltis, Gedeon Richter, MSD, Merck, Pfizer, Roche, Stragen and Mithra Pharmaceuticals. O.H. occasionally serves on advisory boards for Bayer AG and Gedeon Richter and has designed and lectured at educational events for these companies. The other authors have nothing to disclose. O.H. occasionally serves on advisory boards for Bayer AG and Gedeon Richter and has designed and lectured at educational events for these companies. The other authors have nothing to disclose.
Trial registration number: ClinicalTrials.gov NCT02352090.
Trial registration date: 27 January 2015.
Date of first patient’s enrolment: 1 April 2015.
Keywords: acute phase signaling; coagulation; combined contraceptive; complement; estradiol valerate; ethinylestradiol; metabolism; proteome; randomized controlled trial.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Figures

Similar articles
-
Effects of estradiol- and ethinylestradiol-based contraceptives on adrenal steroids: A randomized trial.Contraception. 2022 Dec;116:59-65. doi: 10.1016/j.contraception.2022.08.009. Epub 2022 Sep 7. Contraception. 2022. PMID: 36084710 Clinical Trial.
-
Combined oral contraceptives containing estradiol valerate vs ethinylestradiol on coagulation: A randomized clinical trial.Acta Obstet Gynecol Scand. 2022 Oct;101(10):1102-1111. doi: 10.1111/aogs.14428. Epub 2022 Jul 31. Acta Obstet Gynecol Scand. 2022. PMID: 35909329 Free PMC article. Clinical Trial.
-
Estradiol Valerate in COC Has More Favorable Inflammatory Profile Than Synthetic Ethinyl Estradiol: A Randomized Trial.J Clin Endocrinol Metab. 2020 Jul 1;105(7):dgaa186. doi: 10.1210/clinem/dgaa186. J Clin Endocrinol Metab. 2020. PMID: 32303765 Clinical Trial.
-
Haemostatic and metabolic impact of estradiol pills and drospirenone-containing ethinylestradiol pills vs. levonorgestrel-containing ethinylestradiol pills: A literature review.Eur J Contracept Reprod Health Care. 2015;20(5):329-43. doi: 10.3109/13625187.2015.1050091. Epub 2015 May 26. Eur J Contracept Reprod Health Care. 2015. PMID: 26007631 Review.
-
Metabolic effects of contraceptive steroids.Rev Endocr Metab Disord. 2011 Jun;12(2):63-75. doi: 10.1007/s11154-011-9182-4. Rev Endocr Metab Disord. 2011. PMID: 21538049 Review.
Cited by
-
Metformin and Combined Oral Contraceptive Pills in the Management of Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis.J Clin Endocrinol Metab. 2024 Jan 18;109(2):e817-e836. doi: 10.1210/clinem/dgad465. J Clin Endocrinol Metab. 2024. PMID: 37554096 Free PMC article.
-
Combined oral contraceptives alter ectonucleotidase and adenosine deaminase activities in peripheral blood cells.Purinergic Signal. 2025 Feb 27. doi: 10.1007/s11302-025-10075-w. Online ahead of print. Purinergic Signal. 2025. PMID: 40011299
-
Added administration of estradiol valerate tosurgical treatment for missed abortion in early pregnancy.Am J Transl Res. 2025 Jun 15;17(6):4827-4838. doi: 10.62347/GVKI9955. eCollection 2025. Am J Transl Res. 2025. PMID: 40672574 Free PMC article.
-
Hormones and thrombosis: the dark side of the moon.Blood Transfus. 2024 Jan;22(1):46-54. doi: 10.2450/BloodTransfus.535. Epub 2023 Apr 27. Blood Transfus. 2024. PMID: 37235737 Free PMC article. Review.
-
Extensive modulation of the circulating blood proteome by hormonal contraceptive use across two population studies.Commun Med (Lond). 2025 Apr 22;5(1):131. doi: 10.1038/s43856-025-00856-0. Commun Med (Lond). 2025. PMID: 40263456 Free PMC article.
References
-
- Ågren UM, Anttila M, Mäenpää-Liukko K, Rantala ML, Rautiainen H, Sommer WF, Mommers E.. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in comparison to one containing levonorgestrel and ethinylestradiol on markers of endocrine function. Eur J Contracept Reprod Health Care 2011;16:458–467. - PMC - PubMed
-
- Edwards PA, Kennedy MA, Mak PA.. LXRs; Oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul Pharmacol 2002;38:249–256. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

