Wacker Oxidation of Methylenecyclobutanes: Scope and Selectivity in an Unusual Setting
- PMID: 36416612
- PMCID: PMC10108300
- DOI: 10.1002/anie.202215381
Wacker Oxidation of Methylenecyclobutanes: Scope and Selectivity in an Unusual Setting
Abstract
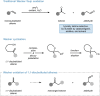
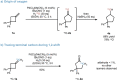
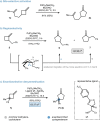
Methylenecyclobutanes are found to undergo Wacker oxidation via a semi-pinacol-type rearrangement. Key to a successful process is the use of tert-butyl nitrite as oxidant, which not only enables efficient catalyst turn-over but also ensures high Markovnikov-selectivity under mild conditions. Thus, cyclopentanones (26 examples) can be accessed in an overall good yield and excellent selectivity (up to 97 % yield, generally >99 : 1 ketone:aldehyde ratio). Stereochemical analysis of the reaction sequence reveals migration aptitudes in line with related 1,2-shifts. By introducing a pyox ligand to palladium, prochiral methylenecyclobutanes can be desymmetrized, thus realizing the first enantioselective Wacker oxidation.
Keywords: 1,2-Rearrangment; Desymmetrization; Strained Rings; Wacker Oxidation.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
TBN as Organic Redox Cocatalyst for Oxidative Tiffeneau-Demjanov-Type Rearrangement Using O2 as Sole Oxidant.Org Lett. 2024 Aug 2;26(30):6454-6458. doi: 10.1021/acs.orglett.4c02264. Epub 2024 Jul 22. Org Lett. 2024. PMID: 39037151
-
Aldehyde-selective Wacker-type oxidation of unbiased alkenes enabled by a nitrite co-catalyst.Angew Chem Int Ed Engl. 2013 Oct 18;52(43):11257-60. doi: 10.1002/anie.201306756. Epub 2013 Sep 13. Angew Chem Int Ed Engl. 2013. PMID: 24039135
-
Catalyst-controlled Wacker-type oxidation: facile access to functionalized aldehydes.J Am Chem Soc. 2014 Jan 22;136(3):890-3. doi: 10.1021/ja411749k. Epub 2014 Jan 13. J Am Chem Soc. 2014. PMID: 24410719
-
Realization of Anti-Markovnikov Selectivity in Pd-Catalyzed Oxidative Acetalization and Wacker-Type Oxidation of Terminal Alkenes.Chem Rec. 2021 Dec;21(12):3458-3469. doi: 10.1002/tcr.202100090. Epub 2021 May 21. Chem Rec. 2021. PMID: 34021681 Review.
-
Sustainable Wacker-Type Oxidations.Angew Chem Int Ed Engl. 2022 Dec 12;61(50):e202211016. doi: 10.1002/anie.202211016. Epub 2022 Oct 26. Angew Chem Int Ed Engl. 2022. PMID: 36164675 Free PMC article. Review.
Cited by
-
Asymmetric Migratory Tsuji-Wacker Oxidation Enables the Enantioselective Synthesis of Hetero- and Isosteric Diarylmethanes.J Am Chem Soc. 2024 Dec 18;146(50):34383-34393. doi: 10.1021/jacs.4c09405. Epub 2024 Dec 7. J Am Chem Soc. 2024. PMID: 39644236 Free PMC article.
-
Immobilization of Trifluoromethyl-Substituted Pyridine-Oxazoline Ligand and Its Application in Asymmetric Continuous Flow Synthesis of Benzosultams.J Org Chem. 2023 Nov 3;88(21):15189-15197. doi: 10.1021/acs.joc.3c01671. Epub 2023 Oct 12. J Org Chem. 2023. PMID: 37823216 Free PMC article.
References
-
- For the discovery of the Wacker oxidation, see:
-
- Smidt J., Hafner W., Jira R., Sedlmeier J., Sieber R., Rüttinger R., Kojer H., Angew. Chem. 1959, 71, 176–182;
-
- Smidt J., Hafner W., Jira R., Sieber R., Sedlmeier J., Sabel A., Angew. Chem. Int. Ed. 1962, 1, 80–88;
- Angew. Chem. 1962, 74, 93–102;
-
- Jira R., Angew. Chem. Int. Ed. 2009, 48, 9034–9037; - PubMed
- Angew. Chem. 2009, 121, 9196–9199.
-
- For pioneering work, see:
Grants and funding
LinkOut - more resources
Full Text Sources

