Allogeneic natural killer cell therapy
- PMID: 36416736
- PMCID: PMC10023727
- DOI: 10.1182/blood.2022016200
Allogeneic natural killer cell therapy
Abstract
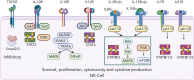
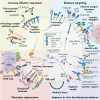
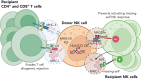
Interest in adoptive cell therapy for treating cancer is exploding owing to early clinical successes of autologous chimeric antigen receptor (CAR) T lymphocyte therapy. However, limitations using T cells and autologous cell products are apparent as they (1) take weeks to generate, (2) utilize a 1:1 donor-to-patient model, (3) are expensive, and (4) are prone to heterogeneity and manufacturing failures. CAR T cells are also associated with significant toxicities, including cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and prolonged cytopenias. To overcome these issues, natural killer (NK) cells are being explored as an alternative cell source for allogeneic cell therapies. NK cells have an inherent ability to recognize cancers, mediate immune functions of killing and communication, and do not induce graft-versus-host disease, cytokine release syndrome, or immune effector cell-associated neurotoxicity syndrome. NK cells can be obtained from blood or cord blood or be derived from hematopoietic stem and progenitor cells or induced pluripotent stem cells, and can be expanded and cryopreserved for off-the-shelf availability. The first wave of point-of-care NK cell therapies led to the current allogeneic NK cell products being investigated in clinical trials with promising preliminary results. Basic advances in NK cell biology and cellular engineering have led to new translational strategies to block inhibition, enhance and broaden target cell recognition, optimize functional persistence, and provide stealth from patients' immunity. This review details NK cell biology, as well as NK cell product manufacturing, engineering, and combination therapies explored in the clinic leading to the next generation of potent, off-the-shelf cellular therapies for blood cancers.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.M.B.-E. and T.A.F. have equity, consulting, and/or potential royalty interest in Wugen Inc. T.A.F. serves on the scientific advisory board of Wugen, Indapta Therapeutics, Orca Bio, and Affimed, and received research funding from HCW Biologics and Affimed. M.T.J. declares no competing financial interests.
Figures




Comment in
-
Introduction to a review series on banked allogeneic immune effector cells.Blood. 2023 Feb 23;141(8):811-812. doi: 10.1182/blood.2023019604. Blood. 2023. PMID: 36608323 No abstract available.
References
-
- Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975;16(2):230–239. - PubMed
-
- Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. 1. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–117. - PubMed
-
- Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18(11):671–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

