Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia
- PMID: 36416819
- PMCID: PMC9685486
- DOI: 10.1001/jamanetworkopen.2022.43691
Analysis of Antibiotic Exposure and Early-Onset Neonatal Sepsis in Europe, North America, and Australia
Abstract
Importance: Appropriate use of antibiotics is life-saving in neonatal early-onset sepsis (EOS), but overuse of antibiotics is associated with antimicrobial resistance and long-term adverse outcomes. Large international studies quantifying early-life antibiotic exposure along with EOS incidence are needed to provide a basis for future interventions aimed at safely reducing neonatal antibiotic exposure.
Objective: To compare early postnatal exposure to antibiotics, incidence of EOS, and mortality among different networks in high-income countries.
Design, setting, and participants: This is a retrospective, cross-sectional study of late-preterm and full-term neonates born between January 1, 2014, and December 31, 2018, in 13 hospital-based or population-based networks from 11 countries in Europe and North America and Australia. The study included all infants born alive at a gestational age greater than or equal to 34 weeks in the participating networks. Data were analyzed from October 2021 to March 2022.
Exposures: Exposure to antibiotics started in the first postnatal week.
Main outcomes and measures: The main outcomes were the proportion of late-preterm and full-term neonates receiving intravenous antibiotics, the duration of antibiotic treatment, the incidence of culture-proven EOS, and all-cause and EOS-associated mortality.
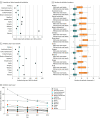
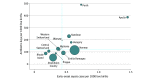
Results: A total of 757 979 late-preterm and full-term neonates were born in the participating networks during the study period; 21 703 neonates (2.86%; 95% CI, 2.83%-2.90%), including 12 886 boys (59.4%) with a median (IQR) gestational age of 39 (36-40) weeks and median (IQR) birth weight of 3250 (2750-3750) g, received intravenous antibiotics during the first postnatal week. The proportion of neonates started on antibiotics ranged from 1.18% to 12.45% among networks. The median (IQR) duration of treatment was 9 (7-14) days for neonates with EOS and 4 (3-6) days for those without EOS. This led to an antibiotic exposure of 135 days per 1000 live births (range across networks, 54-491 days per 1000 live births). The incidence of EOS was 0.49 cases per 1000 live births (range, 0.18-1.45 cases per 1000 live births). EOS-associated mortality was 3.20% (12 of 375 neonates; range, 0.00%-12.00%). For each case of EOS, 58 neonates were started on antibiotics and 273 antibiotic days were administered.
Conclusions and relevance: The findings of this study suggest that antibiotic exposure during the first postnatal week is disproportionate compared with the burden of EOS and that there are wide (up to 9-fold) variations internationally. This study defined a set of indicators reporting on both dimensions to facilitate benchmarking and future interventions aimed at safely reducing antibiotic exposure in early life.
Conflict of interest statement
Figures

Comment in
-
Reducing Antibiotic Exposure at the Beginning of Life.JAMA Netw Open. 2022 Nov 1;5(11):e2243705. doi: 10.1001/jamanetworkopen.2022.43705. JAMA Netw Open. 2022. PMID: 36416827 No abstract available.
References
-
- Versporten A, Zarb P, Caniaux I, et al. ; Global-PPS network . Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619-e629. doi:10.1016/S2214-109X(18)30186-4 - DOI - PubMed
-
- Prusakov P, Goff DA, Wozniak PS, et al. ; Global NEO-ASP Study Group . A global point prevalence survey of antimicrobial use in neonatal intensive care units: the no-more-antibiotics and resistance (NO-MAS-R) study. EClinicalMedicine. 2021;32:100727. doi:10.1016/j.eclinm.2021.100727 - DOI - PMC - PubMed
-
- Hufnagel M, Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H; ARPEC Project Group . High rates of prescribing antimicrobials for prophylaxis in children and neonates: results from the Antibiotic Resistance and Prescribing in European Children Point Prevalence Survey. J Pediatric Infect Dis Soc. 2019;8(2):143-151. doi:10.1093/jpids/piy019 - DOI - PubMed
-
- World Health Organization . World Health Assembly 69. Global action plan on antimicrobial resistance: options for establishing a global development and stewardship framework to support the development, control, distribution and appropriate use of new antimicrobial medicines, diagnostic tools, vaccines and other interventions—report by the Secretariat. 2016. Accessed April 5, 2022. https://apps.who.int/iris/handle/10665/252682

