Exploring the K isotope composition of Göttingen minipig brain regions, and implications for Alzheimer's disease
- PMID: 36416864
- PMCID: PMC9764214
- DOI: 10.1093/mtomcs/mfac090
Exploring the K isotope composition of Göttingen minipig brain regions, and implications for Alzheimer's disease
Abstract
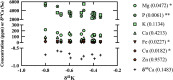
Natural stable metal isotopes have shown utility in differentiation between healthy and diseased brain states (e.g. Alzheimer's disease, AD). While the AD brain accumulates some metals, it purges others, namely K (accompanied by increased serum K, suggesting brain-blood transferal). Here, K isotope compositions of Göttingen minipig brain regions for two AD models at midlife are reported. Results indicate heavy K isotope enrichment where amyloid beta (Aβ) accumulation is observed, and this enrichment correlates with relative K depletion. These results suggest preferential efflux of isotopically light K+ from the brain, a linkage between brain K concentrations and isotope compositions, and linkage to Aβ (previously shown to purge cellular brain K+). Brain K isotope compositions differ from that for serum and brain K is much more abundant than in serum, suggesting that changes in brain K may transfer a measurable K isotope excursion to serum, thereby generating an early AD biomarker.
Keywords: Alzheimer's disease, brain potassium; isotope geochemistry; isotope metallomics; neurodegeneration, porcine model.
© The Author(s) 2022.Published by Oxford University Press.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Adlard P. A., Bush A. I., Metals and Alzheimer's disease, J. Alzheimers Dis., 2006, 10 (2-3), 145–163. - PubMed
-
- Roberts B. R., Doecke J. D., Rembach A., Yevenes L. F., Fowler C. J., McLean C. A., Lind M., Volitakis I., Masters C. L., Bush A. I., Hare D. J., group Ar., Rubidium and potassium levels are altered in Alzheimer's disease brain and blood but not in cerebrospinal fluid, Acta Neuropathol. Commun., 2016, 4 (1), 119. - PMC - PubMed
-
- Balter V., Zazzo A., Moloney A. P., Moynier F., Schmidt O., Monahan F. J., Albarede F., Bodily variability of zinc natural isotope abundances in sheep, Rapid Commun. Mass Spectrom., 2010, 24 (5), 605–612. - PubMed
-
- Balter V., Lamboux A., Zazzo A., Télouk P., Leverrier Y., Marvel J., Moloney A. P., Monahan F. J., Schmidt O., Albarède F., Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation, Metallomics, 2013, 5 (11), 1470–1482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

