Molecular chaperone function of three small heat-shock proteins from a model probiotic species
- PMID: 36417097
- PMCID: PMC9877261
- DOI: 10.1007/s12192-022-01309-6
Molecular chaperone function of three small heat-shock proteins from a model probiotic species
Abstract
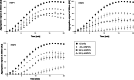
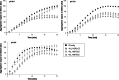
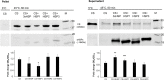
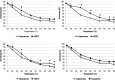
Small heat-shock proteins (sHSP) are ubiquitous ATP-independent chaperones that prevent irreversible aggregation of heat-damaged denaturing proteins. Lactiplantibacillus plantarum is a widespread Gram-positive bacterium with probiotic claims and vast potential for agro-food, biotechnological and biomedical applications. L. plantarum possesses a family of three sHSP, which were previously demonstrated to be involved in its stress tolerance mechanisms. Here, the three L. plantarum sHSP were heterologously expressed, purified and shown to have a chaperone activity in vitro, measuring their capacity to suppress protein aggregation, as assayed spectrophotometrically by light scattering. Their anti-aggregative capacity was found to be differently influenced by pH. Differences were also found relative to their holdase function and their capacity to modulate liposome membrane fluidity, suggesting interplays between them and indicating diversified activities. This is the first study assessing the chaperone action of sHSP from a probiotic model. The different roles of the three sHSP can increase L. plantarum's capabilities to survive the various types of stress characterising the diverse habitats of this highly adaptable species. Reported evidence supports the interest in L. plantarum as one of the model species for bacteria that have three different sHSP-encoding genes in their genomes.
Keywords: Heat stress; Holdase; Lipochaperone; Membrane fluidity; Protein aggregation; sHSP.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Knock out of sHSP genes determines some modifications in the probiotic attitude of Lactiplantibacillus plantarum.Biotechnol Lett. 2021 Mar;43(3):645-654. doi: 10.1007/s10529-020-03041-6. Epub 2020 Nov 6. Biotechnol Lett. 2021. PMID: 33156458 Free PMC article.
-
Production of the small heat shock protein Lo18 from Oenococcus oeni in Lactococcus lactis improves its stress tolerance.Int J Food Microbiol. 2017 Apr 17;247:18-23. doi: 10.1016/j.ijfoodmicro.2016.06.005. Epub 2016 Jun 8. Int J Food Microbiol. 2017. PMID: 27318622
-
The Phenotypic Analysis of Lactobacillus plantarum shsp Mutants Reveals a Potential Role for hsp1 in Cryotolerance.Front Microbiol. 2019 Apr 24;10:838. doi: 10.3389/fmicb.2019.00838. eCollection 2019. Front Microbiol. 2019. PMID: 31114549 Free PMC article.
-
Emerging therapeutic roles of small heat shock protein-derived mini-chaperones and their delivery strategies.Biochimie. 2023 May;208:56-65. doi: 10.1016/j.biochi.2022.12.004. Epub 2022 Dec 12. Biochimie. 2023. PMID: 36521577 Review.
-
Is the lipochaperone activity of sHSP a key to the stress response encoded in its primary sequence?Cell Stress Chaperones. 2023 Jan;28(1):21-33. doi: 10.1007/s12192-022-01308-7. Epub 2022 Nov 11. Cell Stress Chaperones. 2023. PMID: 36367671 Free PMC article. Review.
Cited by
-
Co-encapsulation of E. faecium E297 and γ-aminobutyric acid (GABA): resilience to adverse conditions and application in oat flakes to enhance its functional properties.Braz J Microbiol. 2025 Sep;56(3):1653-1668. doi: 10.1007/s42770-025-01724-8. Epub 2025 Jun 30. Braz J Microbiol. 2025. PMID: 40586955
-
The role of membrane physiology in sHSP Lo18-lipid interaction and lipochaperone activity.Sci Rep. 2024 Jul 24;14(1):17048. doi: 10.1038/s41598-024-67362-6. Sci Rep. 2024. PMID: 39048624 Free PMC article.
-
Characterization and Functional Analysis of Small Heat Shock Protein Genes (Hsp22.2 and Hsp26.7) in Sitodiplosis mosellana Diapause.Insects. 2025 Jun 20;16(7):649. doi: 10.3390/insects16070649. Insects. 2025. PMID: 40725281 Free PMC article.
-
The beauty and complexity of the small heat shock proteins: a report on the proceedings of the fourth workshop on small heat shock proteins.Cell Stress Chaperones. 2023 Nov;28(6):621-629. doi: 10.1007/s12192-023-01360-x. Epub 2023 Jul 18. Cell Stress Chaperones. 2023. PMID: 37462824 Free PMC article. Review.
-
Significant influence of four highly conserved amino-acids in lipochaperon-active sHsps on the structure and functions of the Lo18 protein.Sci Rep. 2023 Nov 3;13(1):19036. doi: 10.1038/s41598-023-46306-6. Sci Rep. 2023. PMID: 37923897 Free PMC article.
References
-
- Balogi Z, Cheregi O, Giese K, Juhasz K, Vierling E, Vass I, Vigh LHI. A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in Synechocystis 6803. J Biol Chem. 2008;283:22983–22991. doi: 10.1074/jbc.M710400200. - DOI - PMC - PubMed

