Polyanhydride Chemistry
- PMID: 36417353
- PMCID: PMC9748945
- DOI: 10.1021/acs.biomac.2c01180
Polyanhydride Chemistry
Abstract
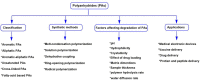
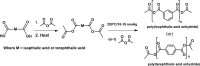
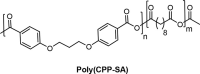
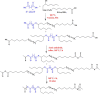
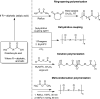
Polyanhydrides (PAs) are a class of synthetic biodegradable polymers employed as controlled drug delivery vehicles. They can be synthesized and scaled up from low-cost starting materials. The structure of PAs can be manipulated synthetically to meet desirable characteristics. PAs are biocompatible, biodegradable, and generate nontoxic metabolites upon degradation, which are easily eliminated from the body. The rate of water penetrating into the polyanhydride (PA) matrix is slower than the anhydride bond cleavage. This phenomenon sets PAs as "surface-eroding drug delivery carriers." Consequently, a variety of PA-based drug delivery carriers in the form of solid implants, pasty injectable formulations, microspheres, nanoparticles, etc. have been developed for the sustained release of small molecule drugs, and vaccines, peptide drugs, and nucleic acid-based active agents. The rate of drug delivery is often controlled by the polymer erosion rate, which is influenced by the polymer structure and composition, crystallinity, hydrophobicity, pH of the release medium, device size, configuration, etc. Owing to the above-mentioned interesting physicochemical and mechanical properties of PAs, the present review focuses on the advancements made in the domain of synthetic biodegradable biomedical PAs for therapeutic delivery applications. Various classes of PAs, their structures, their unique characteristics, their physicochemical and mechanical properties, and factors influencing surface erosion are discussed in detail. The review also summarizes various methods involved in the synthesis of PAs and their utility in the biomedical domain as drug, vaccine, and peptide delivery carriers in different formulations are reviewed.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


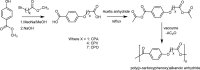






References
-
- Doppalapudi S.; Jain A.; Khan W.; Domb A. J. Biodegradable Polymers-an Overview. Polym. Adv. Technol. 2014, 25, 427–435. 10.1002/pat.3305. - DOI
-
- Guruprasad Reddy P.; Domb A. J. Formation of Micro/nanoparticles and Microspheres from Polyesters by Dispersion Ring-opening Polymerization. Polym. Adv. Technol. 2021, 32, 3835–3856. 10.1002/pat.5476. - DOI
-
- Arun Y.; Ghosh R.; Domb A. J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284.10.1002/adfm.202010284. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

