A gut microbial metabolite of dietary polyphenols reverses obesity-driven hepatic steatosis
- PMID: 36417437
- PMCID: PMC9860326
- DOI: 10.1073/pnas.2202934119
A gut microbial metabolite of dietary polyphenols reverses obesity-driven hepatic steatosis
Abstract
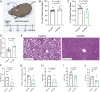
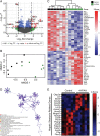
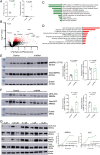
The molecular mechanisms by which dietary fruits and vegetables confer cardiometabolic benefits remain poorly understood. Historically, these beneficial properties have been attributed to the antioxidant activity of flavonoids. Here, we reveal that the host metabolic benefits associated with flavonoid consumption hinge, in part, on gut microbial metabolism. Specifically, we show that a single gut microbial flavonoid catabolite, 4-hydroxyphenylacetic acid (4-HPAA), is sufficient to reduce diet-induced cardiometabolic disease (CMD) burden in mice. The addition of flavonoids to a high fat diet heightened the levels of 4-HPAA within the portal plasma and attenuated obesity, and continuous delivery of 4-HPAA was sufficient to reverse hepatic steatosis. The antisteatotic effect was shown to be associated with the activation of AMP-activated protein kinase α (AMPKα). In a large survey of healthy human gut metagenomes, just over one percent contained homologs of all four characterized bacterial genes required to catabolize flavonols into 4-HPAA. Our results demonstrate the gut microbial contribution to the metabolic benefits associated with flavonoid consumption and underscore the rarity of this process in human gut microbial communities.
Keywords: AMP-activated protein kinase; cardiometabolic disease; flavonoid metabolism; gut microbiome; obesity.
Conflict of interest statement
The authors declare a competing interest. J.C. is a Scientific Advisor for Seed Health, Inc. Z.W. and S.L.H. report being named as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics and therapeutics. S.L.H. reports being a paid consultant for Procter & Gamble, and Zehna Therapeutics, having received research funds from Procter & Gamble, Roche Diagnostics and Zehna therapeutics, and being eligible to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab, a fully owned subsidiary of Quest Diagnostics, Zehna Therapeutics and Procter & Gamble. The other authors declare they have no competing interests.
Figures


References
-
- Pontzer H., Wood B. M., Raichlen D. A., Hunter-gatherers as models in public health. Obesity Rev. 19, 24–35 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

