Manipulating polydispersity of lens β-crystallins using divalent cations demonstrates evidence of calcium regulation
- PMID: 36417439
- PMCID: PMC9860307
- DOI: 10.1073/pnas.2212051119
Manipulating polydispersity of lens β-crystallins using divalent cations demonstrates evidence of calcium regulation
Abstract
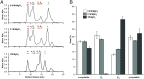
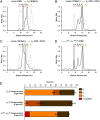
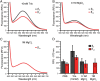
Crystallins comprise the protein-rich tissue of the eye lens. Of the three most common vertebrate subtypes, β-crystallins exhibit the widest degree of polydispersity due to their complex multimerization properties in situ. While polydispersity enables precise packing densities across the concentration gradient of the lens for vision, it is unclear why there is such a high degree of structural complexity within the β-crystallin subtype and what the role of this feature is in the lens. To investigate this, we first characterized β-crystallin polydispersity and then established a method to dynamically disrupt it in a process that is dependent on isoform composition and the presence of divalent cationic salts (CaCl2 or MgCl2). We used size-exclusion chromatography together with dynamic light scattering and mass spectrometry to show how high concentrations of divalent cations dissociate β-crystallin oligomers, reduce polydispersity, and shift the overall protein surface charge-properties that can be reversed when salts are removed. While the direct, physiological relevance of these divalent cations in the lens is still under investigation, our results support that specific isoforms of β-crystallin modulate polydispersity through multiple chemical equilibria and that this native state is disrupted by cation binding. This dynamic process may be essential to facilitating the molecular packing and optical function of the lens.
Keywords: assembly; divalent cations; lens; polydispersity; β-crystallin.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Divalent Cations and the Divergence of βγ-Crystallin Function.Biochemistry. 2019 Nov 12;58(45):4505-4518. doi: 10.1021/acs.biochem.9b00507. Epub 2019 Nov 1. Biochemistry. 2019. PMID: 31647219 Free PMC article.
-
Eye lens β-crystallins are predicted by native ion mobility-mass spectrometry and computations to form compact higher-ordered heterooligomers.Structure. 2023 Sep 7;31(9):1052-1064.e3. doi: 10.1016/j.str.2023.06.013. Epub 2023 Jul 14. Structure. 2023. PMID: 37453416 Free PMC article.
-
Calcium-binding to lens betaB2- and betaA3-crystallins suggests that all beta-crystallins are calcium-binding proteins.FEBS J. 2007 Aug;274(16):4135-47. doi: 10.1111/j.1742-4658.2007.05941.x. Epub 2007 Jul 25. FEBS J. 2007. PMID: 17651443
-
Lens crystallins of invertebrates--diversity and recruitment from detoxification enzymes and novel proteins.Eur J Biochem. 1996 Feb 1;235(3):449-65. doi: 10.1111/j.1432-1033.1996.00449.x. Eur J Biochem. 1996. PMID: 8654388 Review.
-
Ca2+ and βγ-crystallins: An affair that did not last?Biochim Biophys Acta. 2016 Jan;1860(1 Pt B):299-303. doi: 10.1016/j.bbagen.2015.06.012. Epub 2015 Jul 2. Biochim Biophys Acta. 2016. PMID: 26145580 Review.
Cited by
-
Oxidative Stress in Cataract Formation: Is There a Treatment Approach on the Horizon?Antioxidants (Basel). 2024 Oct 16;13(10):1249. doi: 10.3390/antiox13101249. Antioxidants (Basel). 2024. PMID: 39456502 Free PMC article. Review.
-
Design and Characterization of Model Systems that Promote and Disrupt Transparency of Vertebrate Crystallins In Vitro.Adv Sci (Weinh). 2023 Dec;10(35):e2303279. doi: 10.1002/advs.202303279. Epub 2023 Oct 28. Adv Sci (Weinh). 2023. PMID: 37897315 Free PMC article.
References
-
- Fagerholm P. P., Philipson B. T., Lindstrom B., Normal human lens-the distribution of protein. Exp. Eye Res. 33, 615–620 (1981). - PubMed
-
- Pierscionek B., Augusteyn R. C., Protein distribution patterns in concentric layers from single bovine lenses: Changes with development and ageing. Curr. Eye Res. 7, 11–23 (1988). - PubMed
-
- Trokel S., The physical basis for transparency of the crystalline lens. Invest. Ophthalmol. 1, 493–501 (1962). - PubMed
-
- Pierscionek B. K., Chan D. Y., Refractive index gradient of human lenses. Optom. Vis. Sci. 66, 822–829 (1989). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

