Systematic assessment of the replicability and generalizability of preclinical findings: Impact of protocol harmonization across laboratory sites
- PMID: 36417471
- PMCID: PMC9728859
- DOI: 10.1371/journal.pbio.3001886
Systematic assessment of the replicability and generalizability of preclinical findings: Impact of protocol harmonization across laboratory sites
Abstract
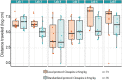
The influence of protocol standardization between laboratories on their replicability of preclinical results has not been addressed in a systematic way. While standardization is considered good research practice as a means to control for undesired external noise (i.e., highly variable results), some reports suggest that standardized protocols may lead to idiosyncratic results, thus undermining replicability. Through the EQIPD consortium, a multi-lab collaboration between academic and industry partners, we aimed to elucidate parameters that impact the replicability of preclinical animal studies. To this end, 3 experimental protocols were implemented across 7 laboratories. The replicability of results was determined using the distance travelled in an open field after administration of pharmacological compounds known to modulate locomotor activity (MK-801, diazepam, and clozapine) in C57BL/6 mice as a worked example. The goal was to determine whether harmonization of study protocols across laboratories improves the replicability of the results and whether replicability can be further improved by systematic variation (heterogenization) of 2 environmental factors (time of testing and light intensity during testing) within laboratories. Protocols were tested in 3 consecutive stages and differed in the extent of harmonization across laboratories and standardization within laboratories: stage 1, minimally aligned across sites (local protocol); stage 2, fully aligned across sites (harmonized protocol) with and without systematic variation (standardized and heterogenized cohort); and stage 3, fully aligned across sites (standardized protocol) with a different compound. All protocols resulted in consistent treatment effects across laboratories, which were also replicated within laboratories across the different stages. Harmonization of protocols across laboratories reduced between-lab variability substantially compared to each lab using their local protocol. In contrast, the environmental factors chosen to introduce systematic variation within laboratories did not affect the behavioral outcome. Therefore, heterogenization did not reduce between-lab variability further compared to the harmonization of the standardized protocol. Altogether, these findings demonstrate that subtle variations between lab-specific study protocols may introduce variation across independent replicate studies even after protocol harmonization and that systematic heterogenization of environmental factors may not be sufficient to account for such between-lab variation. Differences in replicability of results within and between laboratories highlight the ubiquity of study-specific variation due to between-lab variability, the importance of transparent and fine-grained reporting of methodologies and research protocols, and the importance of independent study replication.
Copyright: © 2022 Arroyo-Araujo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

