CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis
- PMID: 36417485
- PMCID: PMC10199725
- DOI: 10.1126/scitranslmed.abp8309
CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis
Abstract
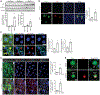
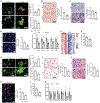
Necroptosis contributes to hepatocyte death in nonalcoholic steatohepatitis (NASH), but the fate and roles of necroptotic hepatocytes (necHCs) in NASH remain unknown. We show here that the accumulation of necHCs in human and mouse NASH liver is associated with an up-regulation of the "don't-eat-me" ligand CD47 on necHCs, but not on apoptotic hepatocytes, and an increase in the CD47 receptor SIRPα on liver macrophages, consistent with impaired macrophage-mediated clearance of necHCs. In vitro, necHC clearance by primary liver macrophages was enhanced by treatment with either anti-CD47 or anti-SIRPα. In a proof-of-concept mouse model of inducible hepatocyte necroptosis, anti-CD47 antibody treatment increased necHC uptake by liver macrophages and inhibited markers of hepatic stellate cell (HSC) activation, which is responsible for liver fibrogenesis. Treatment of two mouse models of diet-induced NASH with anti-CD47, anti-SIRPα, or AAV8-H1-shCD47 to silence CD47 in hepatocytes increased the uptake of necHC by liver macrophages and decreased markers of HSC activation and liver fibrosis. Anti-SIRPα treatment avoided the adverse effect of anemia found in anti-CD47-treated mice. These findings provide evidence that impaired clearance of necHCs by liver macrophages due to CD47-SIRPα up-regulation contributes to fibrotic NASH, and suggest therapeutic blockade of the CD47-SIRPα axis as a strategy to decrease the accumulation of necHCs in NASH liver and dampen the progression of hepatic fibrosis.
Conflict of interest statement
Figures

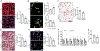



References
-
- Younossi ZM, Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol 70, 531–544 (2019). - PubMed
-
- Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA, The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol 7, 851–861 (2022). - PubMed
-
- Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, Paradis V, Bedossa P, Aldridge Whitehead JM, Labourdette A, Miette V, Neubauer S, Fournier C, Paredes AH, Alkhouri N, Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J. Hepatol 75, 284–291 (2021). - PubMed
-
- Sanyal AJ, Van Natta ML, Clark J, Neuschwander-Tetri BA, Diehl A, Dasarathy S, Loomba R, Chalasani N, Kowdley K, Hameed B, Wilson LA, Yates KP, Belt P, Lazo M, Kleiner DE, Behling C, Tonascia J, Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med 385, 1559–1569 (2021). - PMC - PubMed
-
- Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F, Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149, 389–397.e10 (2015). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 DK115778/DK/NIDDK NIH HHS/United States
- R00 HL130574/HL/NHLBI NIH HHS/United States
- S10 RR025686/RR/NCRR NIH HHS/United States
- P30 DK132710/DK/NIDDK NIH HHS/United States
- R00 HL145131/HL/NHLBI NIH HHS/United States
- T32 HL007343/HL/NHLBI NIH HHS/United States
- R01 DK116620/DK/NIDDK NIH HHS/United States
- HHSN276201200017C/LM/NLM NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- R01 HL151611/HL/NHLBI NIH HHS/United States
- R35 GM147269/GM/NIGMS NIH HHS/United States
- UL1 TR001873/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

