Macrophage acetyl-CoA carboxylase regulates acute inflammation through control of glucose and lipid metabolism
- PMID: 36417534
- PMCID: PMC9683712
- DOI: 10.1126/sciadv.abq1984
Macrophage acetyl-CoA carboxylase regulates acute inflammation through control of glucose and lipid metabolism
Abstract
Acetyl-CoA carboxylase (ACC) regulates lipid synthesis; however, its role in inflammatory regulation in macrophages remains unclear. We generated mice that are deficient in both ACC isoforms in myeloid cells. ACC deficiency altered the lipidomic, transcriptomic, and bioenergetic profile of bone marrow-derived macrophages, resulting in a blunted response to proinflammatory stimulation. In response to lipopolysaccharide (LPS), ACC is required for the early metabolic switch to glycolysis and remodeling of the macrophage lipidome. ACC deficiency also resulted in impaired macrophage innate immune functions, including bacterial clearance. Myeloid-specific deletion or pharmacological inhibition of ACC in mice attenuated LPS-induced expression of proinflammatory cytokines interleukin-6 (IL-6) and IL-1β, while pharmacological inhibition of ACC increased susceptibility to bacterial peritonitis in wild-type mice. Together, we identify a critical role for ACC in metabolic regulation of the innate immune response in macrophages, and thus a clinically relevant, unexpected consequence of pharmacological ACC inhibition.
Figures



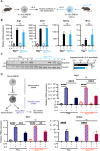



References
-
- Freemerman A. J., Zhao L., Pingili A. K., Teng B., Cozzo A. J., Fuller A. M., Johnson A. R., Milner J. J., Lim M. F., Galanko J. A., Beck M. A., Bear J. E., Rotty J. D., Bezavada L., Smallwood H. S., Puchowicz M. A., Liu J., Locasale J. W., Lee D. P., Bennett B. J., Abel E. D., Rathmell J. C., Makowski L.,Myeloid Slc2a1-deficient murine model revealed macrophage activation and metabolic phenotype are fueled by GLUT1. J. Immunol. 202,1265–1286 (2019). - PMC - PubMed
-
- Tannahill G. M., Curtis A. M., Adamik J., Palsson-McDermott E. M., McGettrick A. F., Goel G., Frezza C., Bernard N. J., Kelly B., Foley N. H., Zheng L., Gardet A., Tong Z., Jany S. S., Corr S. C., Haneklaus M., Caffrey B. E., Pierce K., Walmsley S., Beasley F. C., Cummins E., Nizet V., Whyte M., Taylor C. T., Lin H., Masters S. L., Gottlieb E., Kelly V. P., Clish C., Auron P. E., Xavier R. J., O’Neill L. A. J.,Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496,238–242 (2013). - PMC - PubMed
-
- Huang S. C.-C., Everts B., Ivanova Y., O’Sullivan D., Nascimento M., Smith A. M., Beatty W., Love-Gregory L., Lam W. Y., O’Neill C. M., Yan C., Du H., Abumrad N. A., Urban J. F. Jr., Artyomov M. N., Pearce E. L., Pearce E. J.,Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat. Immunol. 15,846–855 (2014). - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

