Circulating c-Met-Expressing Memory T Cells Define Cardiac Autoimmunity
- PMID: 36417924
- PMCID: PMC9770129
- DOI: 10.1161/CIRCULATIONAHA.121.055610
Circulating c-Met-Expressing Memory T Cells Define Cardiac Autoimmunity
Abstract
Background: Autoimmunity is increasingly recognized as a key contributing factor in heart muscle diseases. The functional features of cardiac autoimmunity in humans remain undefined because of the challenge of studying immune responses in situ. We previously described a subset of c-mesenchymal epithelial transition factor (c-Met)-expressing (c-Met+) memory T lymphocytes that preferentially migrate to cardiac tissue in mice and humans.
Methods: In-depth phenotyping of peripheral blood T cells, including c-Met+ T cells, was undertaken in groups of patients with inflammatory and noninflammatory cardiomyopathies, patients with noncardiac autoimmunity, and healthy controls. Validation studies were carried out using human cardiac tissue and in an experimental model of cardiac inflammation.
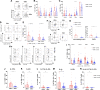
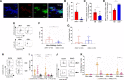
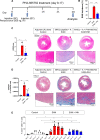
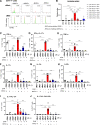
Results: We show that c-Met+ T cells are selectively increased in the circulation and in the myocardium of patients with inflammatory cardiomyopathies. The phenotype and function of c-Met+ T cells are distinct from those of c-Met-negative (c-Met-) T cells, including preferential proliferation to cardiac myosin and coproduction of multiple cytokines (interleukin-4, interleukin-17, and interleukin-22). Furthermore, circulating c-Met+ T cell subpopulations in different heart muscle diseases identify distinct and overlapping mechanisms of heart inflammation. In experimental autoimmune myocarditis, elevations in autoantigen-specific c-Met+ T cells in peripheral blood mark the loss of immune tolerance to the heart. Disease development can be halted by pharmacologic c-Met inhibition, indicating a causative role for c-Met+ T cells.
Conclusions: Our study demonstrates that the detection of circulating c-Met+ T cells may have use in the diagnosis and monitoring of adaptive cardiac inflammation and definition of new targets for therapeutic intervention when cardiac autoimmunity causes or contributes to progressive cardiac injury.
Keywords: T-lymphocytes; cardiac myosins; cardiomyopathies; heart; hepatocyte growth factor; humans; inflammation; mice; myocarditis; therapeutics.
Figures


Comment in
-
Straight to the Heart: T Cells That Specifically Target Cardiac Tissue.Circulation. 2022 Dec 20;146(25):1946-1949. doi: 10.1161/CIRCULATIONAHA.122.061324. Epub 2022 Dec 19. Circulation. 2022. PMID: 36534731 No abstract available.
References
-
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, et al. ; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a–2648d. doi: 10.1093/eurheartj/eht210 - PubMed
-
- Gallagher S, Jones DA, Anand V, Mohiddin S. Diagnosis and management of patients with acute cardiac symptoms, troponin elevation and culprit-free angiograms. Heart. 2012;98:974–981. doi: 10.1136/heartjnl-2011-301121 - PubMed
-
- Heidecker B, Ruedi G, Baltensperger N, Gresser E, Kottwitz J, Berg J, Manka R, Landmesser U, Luscher TF, Patriki D. Systematic use of cardiac magnetic resonance imaging in MINOCA led to a five-fold increase in the detection rate of myocarditis: a retrospective study. Swiss Med Wkly. 2019;149:w20098. doi: 10.4414/smw.2019.20098 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

