Fulminant Myocarditis and Acute Appendicitis after COVID-19 Vaccination
- PMID: 36418095
- PMCID: PMC9970817
- DOI: 10.2169/internalmedicine.0680-22
Fulminant Myocarditis and Acute Appendicitis after COVID-19 Vaccination
Abstract
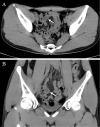
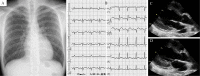
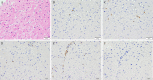
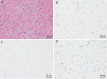
A 19-year-old Japanese man was hospitalized for cardiogenic shock 28 days after receiving a second dose of the coronavirus disease 2019 (COVID-19) mRNA-1273 vaccine. He had had a high fever for three days with vomiting and abdominal pain before arriving at our hospital. The patient visited a local hospital and was diagnosed with heart failure and acute appendicitis. An endomyocardial biopsy specimen showed myocarditis. Thereafter, Impella CP left ventricular assist device implantation and venoarterial peripheral extracorporeal membranous oxygenation were initiated immediately along with inotropic support and steroid pulse therapy. Given these findings, he was finally diagnosed with multiple inflammatory syndrome and fulminant myocarditis.
Keywords: biopsy; cytokine; inflammation.
Conflict of interest statement
Figures

Similar articles
-
Fulminant Myocarditis 24 Days after Coronavirus Disease Messenger Ribonucleic Acid Vaccination.Intern Med. 2022 Aug 1;61(15):2319-2325. doi: 10.2169/internalmedicine.9800-22. Epub 2022 May 31. Intern Med. 2022. PMID: 35650138 Free PMC article.
-
Fulminant necrotizing eosinophilic myocarditis after COVID-19 vaccination survived with mechanical circulatory support.ESC Heart Fail. 2022 Aug;9(4):2732-2737. doi: 10.1002/ehf2.13962. Epub 2022 May 26. ESC Heart Fail. 2022. PMID: 35616026 Free PMC article.
-
Short-term use of "ECMELLA" in the context of fulminant eosinophilic myocarditis with cardiogenic shock.BMC Cardiovasc Disord. 2020 Dec 10;20(1):519. doi: 10.1186/s12872-020-01808-3. BMC Cardiovasc Disord. 2020. PMID: 33302874 Free PMC article.
-
Severe COVID-19-associated myocarditis with cardiogenic shock - management with assist devices - a case report & review.BMC Anesthesiol. 2022 Dec 12;22(1):385. doi: 10.1186/s12871-022-01890-4. BMC Anesthesiol. 2022. PMID: 36503438 Free PMC article. Review.
-
Mechanical Unloading by Fulminant Myocarditis: LV-IMPELLA, ECMELLA, BI-PELLA, and PROPELLA Concepts.J Cardiovasc Transl Res. 2019 Apr;12(2):116-123. doi: 10.1007/s12265-018-9820-2. Epub 2018 Aug 6. J Cardiovasc Transl Res. 2019. PMID: 30084076 Free PMC article. Review.
Cited by
-
SARS-CoV-2 vaccines and myocarditis.Clin Med (Lond). 2023 Sep;23(5):495-502. doi: 10.7861/clinmed.2023-0049. Epub 2023 Sep 29. Clin Med (Lond). 2023. PMID: 37775180 Free PMC article. Review.
-
Clinical Application of Extracorporeal Membrane Oxygenation in the Treatment of Fulminant Myocarditis.Rev Cardiovasc Med. 2024 Mar 26;25(4):114. doi: 10.31083/j.rcm2504114. eCollection 2024 Apr. Rev Cardiovasc Med. 2024. PMID: 39076539 Free PMC article. Review.
-
Acute abdomen following COVID-19 vaccination: a systematic review.Clin Exp Vaccine Res. 2024 Jan;13(1):42-53. doi: 10.7774/cevr.2024.13.1.42. Epub 2024 Jan 31. Clin Exp Vaccine Res. 2024. PMID: 38362368 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

