Disrupting circadian control of peripheral myogenic reactivity mitigates cardiac injury following myocardial infarction
- PMID: 36418171
- PMCID: PMC10262184
- DOI: 10.1093/cvr/cvac174
Disrupting circadian control of peripheral myogenic reactivity mitigates cardiac injury following myocardial infarction
Abstract
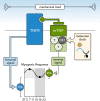
Aims: Circadian rhythms orchestrate important functions in the cardiovascular system: the contribution of microvascular rhythms to cardiovascular disease progression/severity is unknown. This study hypothesized that (i) myogenic reactivity in skeletal muscle resistance arteries is rhythmic and (ii) disrupting this rhythmicity would alter cardiac injury post-myocardial infarction (MI).
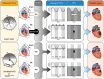
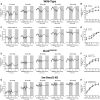
Methods and results: Cremaster skeletal muscle resistance arteries were isolated and assessed using standard pressure myography. Circadian rhythmicity was globally disrupted with the ClockΔ19/Δ19 mutation or discretely through smooth muscle cell-specific Bmal1 deletion (Sm-Bmal1 KO). Cardiac structure and function were determined by echocardiographic, hemodynamic and histological assessments. Myogenic reactivity in cremaster muscle resistance arteries is rhythmic. This rhythm is putatively mediated by the circadian modulation of a mechanosensitive signalosome incorporating tumour necrosis factor and casein kinase 1. Following left anterior descending coronary artery ligation, myogenic responsiveness is locked at the circadian maximum, although circadian molecular clock gene expression cycles normally. Disrupting the molecular clock abolishes myogenic rhythmicity: myogenic tone is suspended at the circadian minimum and is no longer augmented by MI. The reduced myogenic tone in ClockΔ19/Δ19 mice and Sm-Bmal1 KO mice associates with reduced total peripheral resistance (TPR), improved cardiac function and reduced infarct expansion post-MI.
Conclusions: Augmented microvascular constriction aggravates cardiac injury post-MI. Following MI, skeletal muscle resistance artery myogenic reactivity increases specifically within the rest phase, when TPR would normally decline. Disrupting the circadian clock interrupts the MI-induced augmentation in myogenic reactivity: therapeutics targeting the molecular clock, therefore, may be useful for improving MI outcomes.
Keywords: Casein kinase 1; Systemic hemodynamics; Total peripheral resistance; Tumour necrosis factor; Vascular smooth muscle cells.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: J.T.K. and D.L. have consulted for Qanatpharma AG (Stans, Switzerland) and Aphaia Pharma AG (Zug, Switzerland) within the last 36 months. J.H. is an employee of Qanatpharma AG. S.S.B. is a founder and executive board member of Qanatpharma AG and Aphaia Pharma AG. Aphaia Pharma AG had no financial or intellectual involvement in this article. Qanatpharma AG played no role in study design, data collection/analysis, decision to publish, or the preparation of the publication. Aside from J.T.K., D.L., J.H., and SSB, none of the other authors have relationships to industry.
Figures




References
-
- Drexler H. Changes in the peripheral circulation in heart failure. Curr Opin Cardiol 1995;10:268–273. - PubMed
-
- Freis ED, Schnaper HW, Johnson RL, Schreiner GE. Hemodynamic alterations in acute myocardial infarction. I. Cardiac output, mean arterial pressure, total peripheral resistance, central and total blood volumes, venous pressure and average circulation time. J Clin Invest 1952;31:131–140. - PMC - PubMed
-
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990;81:1161–1172. - PubMed
-
- Eaton LW, Weiss JL, Bulkley BH, Garrison JB, Weisfeldt ML. Regional cardiac dilatation after acute myocardial infarction: recognition by two-dimensional echocardiography. N Engl J Med 1979;300:57–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

