Resonant Inelastic Soft X-ray Scattering and X-ray Emission Spectroscopy of Solid Proline and Proline Solutions
- PMID: 36418225
- PMCID: PMC9744097
- DOI: 10.1021/acs.jpcb.2c06557
Resonant Inelastic Soft X-ray Scattering and X-ray Emission Spectroscopy of Solid Proline and Proline Solutions
Abstract
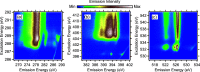
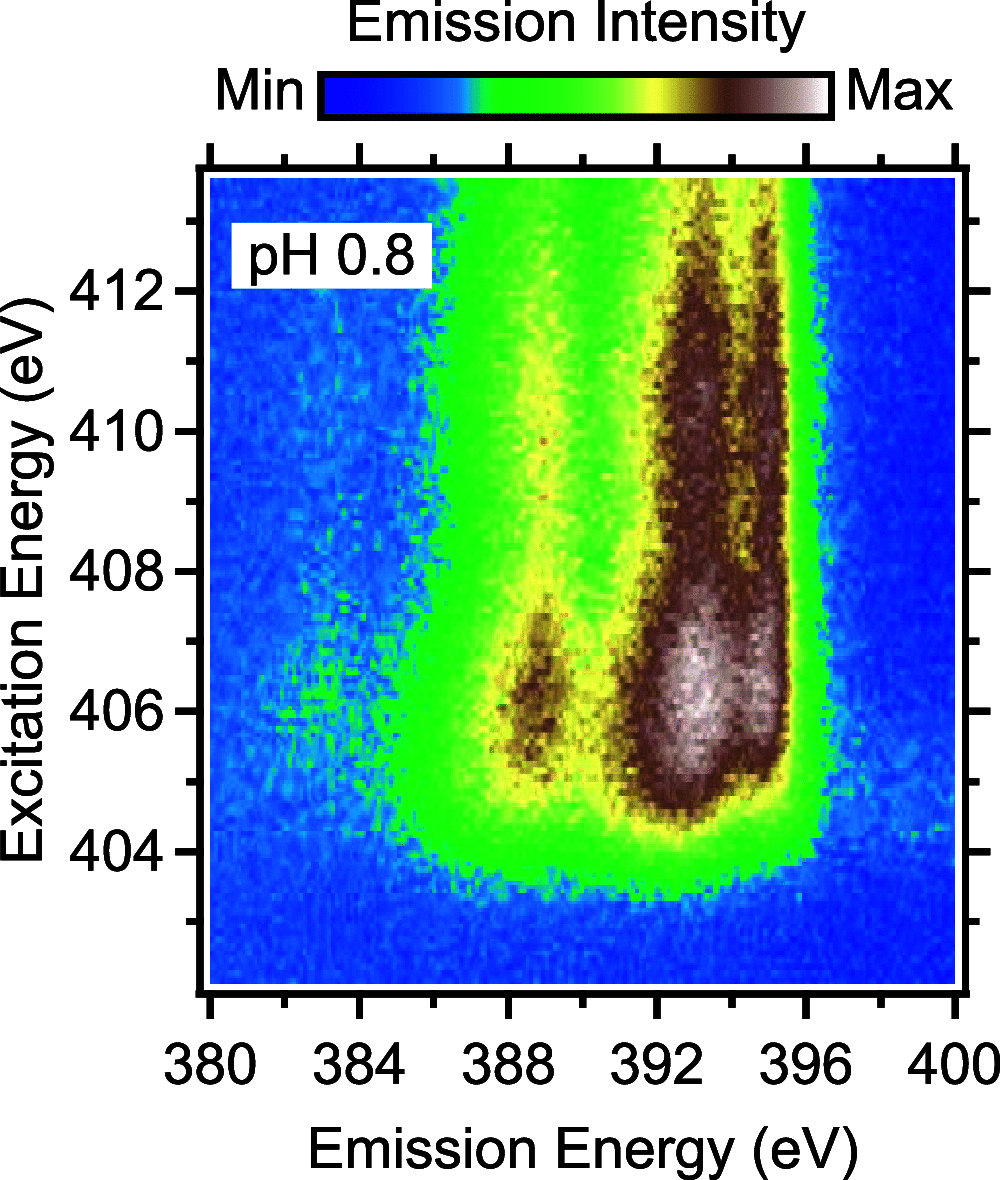
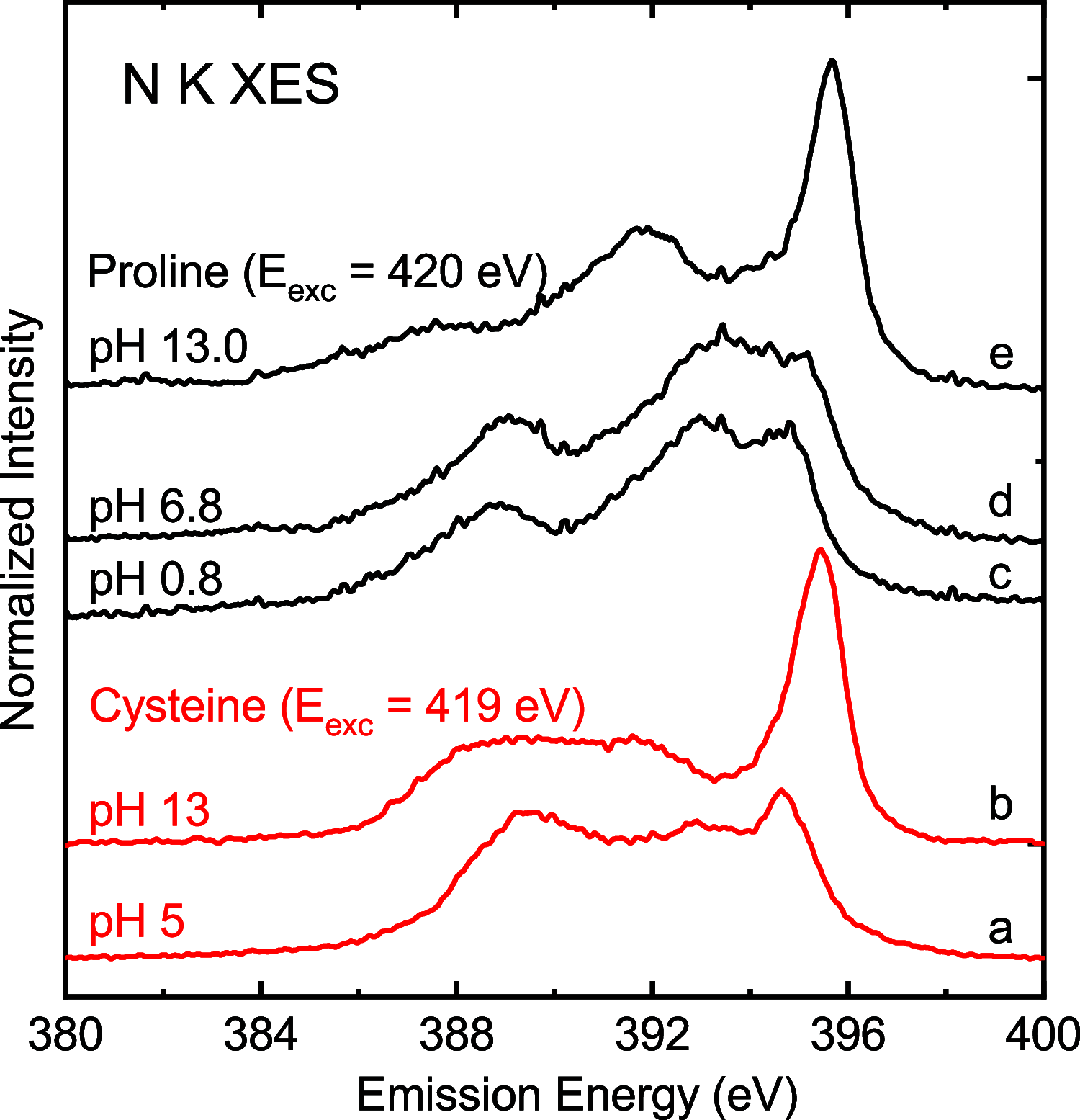
The amino group of proline is part of a pyrrolidine ring, which makes it unique among the proteinogenic amino acids. To unravel its full electronic structure, proline in solid state and aqueous solution is investigated using X-ray emission spectroscopy and resonant inelastic soft X-ray scattering. By controlling the pH value of the solution, proline is studied in its cationic, zwitterionic, and anionic configurations. The spectra are analyzed within a "building-block principle" by comparing with suitable reference molecules, i.e., acetic acid, cysteine, and pyrrolidine, as well as with spectral calculations based on density functional theory. We find that the electronic structure of the carboxyl group of proline is very similar to that of other amino acids as well as acetic acid. In contrast, the electronic structure of the amino group is significantly different and strongly influenced by the ring structure of proline.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


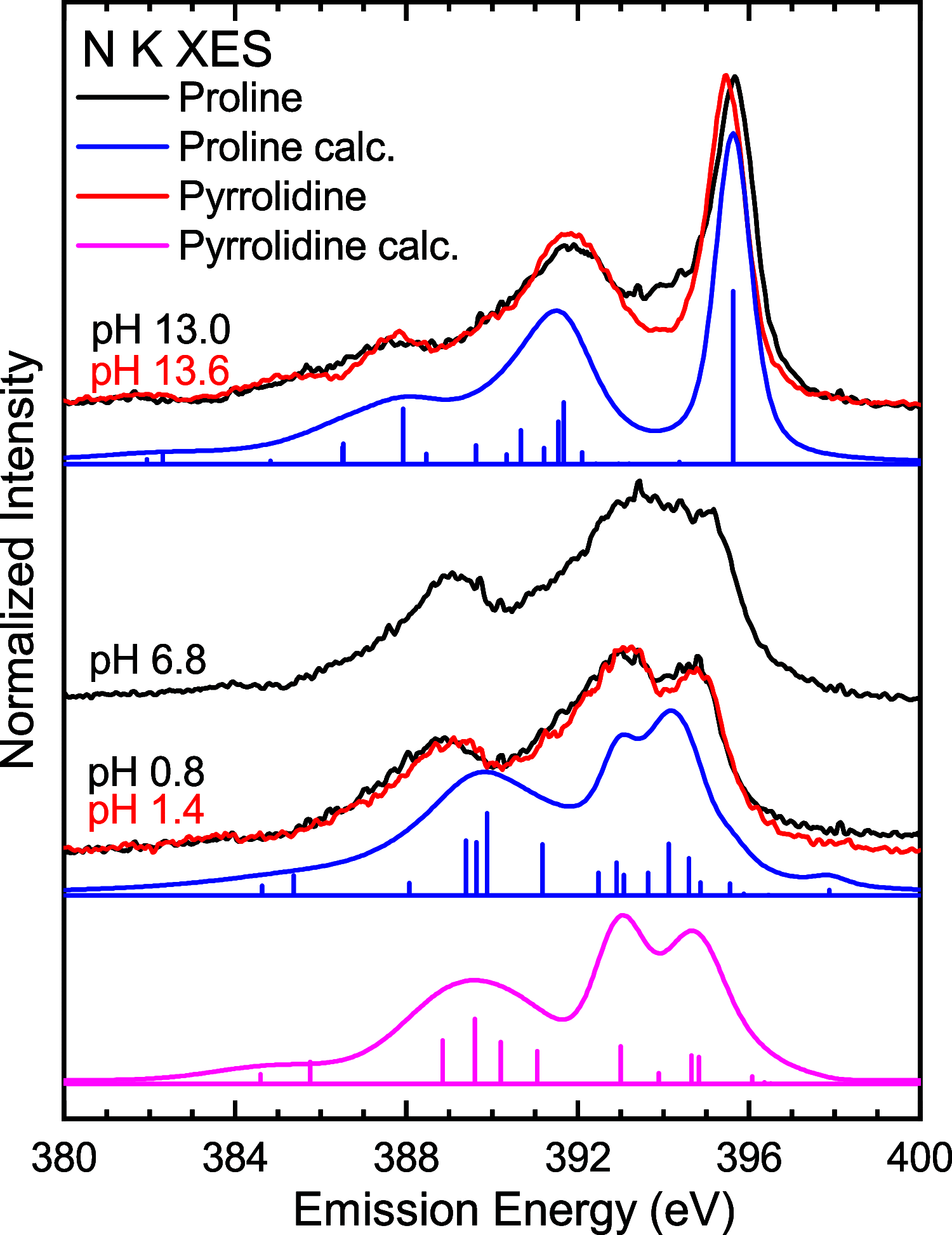
References
-
- Wagner I.; Musso H. New Naturally Occurring Amino Acids. Angew. Chem., Int. Ed. 1983, 22, 816–828. 10.1002/anie.198308161. - DOI
-
- Cavalleri O.; Gonella G.; Terreni S.; Vignolo M.; Floreano L.; Morgante A.; Canepa M.; Rolandi R. High Resolution X-ray Photoelectron Spectroscopy of L-Cysteine Self-Assembled Films. Phys. Chem. Chem. Phys. 2004, 6, 4042–4046. 10.1039/b405516k. - DOI
-
- Kamada M.; Sugiyama H.; Takahashi K.; Azuma J.; Kitajima S.; Ogawa K.; Sumimoto M.; Hori K.; Fujimoto H. Photoelectron Spectroscopic Study of Electronic Structures of L-Cysteine. J. Phys. Soc. Jpn. 2010, 79, 03470910.1143/JPSJ.79.034709. - DOI

