Structure- and computational-aided engineering of an oxidase to produce isoeugenol from a lignin-derived compound
- PMID: 36418310
- PMCID: PMC9684555
- DOI: 10.1038/s41467-022-34912-3
Structure- and computational-aided engineering of an oxidase to produce isoeugenol from a lignin-derived compound
Abstract
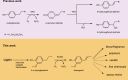
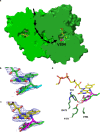
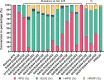
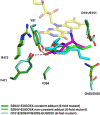
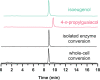
Various 4-alkylphenols can be easily obtained through reductive catalytic fractionation of lignocellulosic biomass. Selective dehydrogenation of 4-n-propylguaiacol results in the formation of isoeugenol, a valuable flavor and fragrance molecule and versatile precursor compound. Here we present the engineering of a bacterial eugenol oxidase to catalyze this reaction. Five mutations, identified from computational predictions, are first introduced to render the enzyme more thermostable. Other mutations are then added and analyzed to enhance chemoselectivity and activity. Structural insight demonstrates that the slow catalytic activity of an otherwise promising enzyme variant is due the formation of a slowly-decaying covalent substrate-flavin cofactor adduct that can be remedied by targeted residue changes. The final engineered variant comprises eight mutations, is thermostable, displays good activity and acts as a highly chemoselective 4-n-propylguaiacol oxidase. We lastly use our engineered biocatalyst in an illustrative preparative reaction at gram-scale. Our findings show that a natural enzyme can be redesigned into a tailored biocatalyst capable of valorizing lignin-based monophenols.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Koelewijn SF, et al. Sustainable bisphenols from renewable softwood lignin feedstock for polycarbonates and cyanate ester resins. Green. Chem. 2017;19:2561–2570. doi: 10.1039/C7GC00776K. - DOI
-
- Tramontina R, et al. Consolidated production of coniferol and other high-value aromatic alcohols directly from lignocellulosic biomass. Green. Chem. 2020;22:144–152. doi: 10.1039/C9GC02359C. - DOI
-
- Renders T, Van den Bosch S, Koelewijn SF, Schutyser W, Sels BF. Lignin-first biomass fractionation: the advent of active stabilisation strategies. Energy Environ. Sci. 2017;10:1551–1557. doi: 10.1039/C7EE01298E. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

