A charged diatomic triple-bonded U≡N species trapped in C82 fullerene cages
- PMID: 36418311
- PMCID: PMC9684569
- DOI: 10.1038/s41467-022-34651-5
A charged diatomic triple-bonded U≡N species trapped in C82 fullerene cages
Abstract
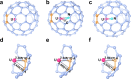
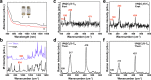
Actinide diatomic molecules are ideal models to study elusive actinide multiple bonds, but most of these diatomic molecules have so far only been studied in solid inert gas matrices. Herein, we report a charged U≡N diatomic species captured in fullerene cages and stabilized by the U-fullerene coordination interaction. Two diatomic clusterfullerenes, viz. UN@Cs(6)-C82 and UN@C2(5)-C82, were successfully synthesized and characterized. Crystallographic analysis reveals U-N bond lengths of 1.760(7) and 1.760(20) Å in UN@Cs(6)-C82 and UN@C2(5)-C82. Moreover, U≡N was found to be immobilized and coordinated to the fullerene cages at 100 K but it rotates inside the cage at 273 K. Quantum-chemical calculations show a (UN)2+@(C82)2- electronic structure with formal +5 oxidation state (f1) of U and unambiguously demonstrate the presence of a U≡N bond in the clusterfullerenes. This study constitutes an approach to stabilize fundamentally important actinide multiply bonded species.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
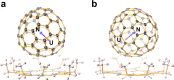



References
-
- King DM, Liddle ST. Progress in molecular uranium-nitride chemistry. Coord. Chem. Rev. 2014;266-267:2–15. doi: 10.1016/j.ccr.2013.06.013. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

