The systemic renin-angiotensin system in COVID-19
- PMID: 36418458
- PMCID: PMC9684482
- DOI: 10.1038/s41598-022-24628-1
The systemic renin-angiotensin system in COVID-19
Abstract
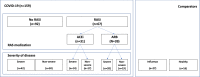
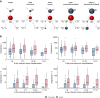
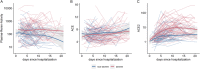
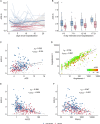
SARS-CoV-2 gains cell entry via angiotensin-converting enzyme (ACE) 2, a membrane-bound enzyme of the "alternative" (alt) renin-angiotensin system (RAS). ACE2 counteracts angiotensin II by converting it to potentially protective angiotensin 1-7. Using mass spectrometry, we assessed key metabolites of the classical RAS (angiotensins I-II) and alt-RAS (angiotensins 1-7 and 1-5) pathways as well as ACE and ACE2 concentrations in 159 patients hospitalized with COVID-19, stratified by disease severity (severe, n = 76; non-severe: n = 83). Plasma renin activity (PRA-S) was calculated as the sum of RAS metabolites. We estimated ACE activity using the angiotensin II:I ratio (ACE-S) and estimated systemic alt-RAS activation using the ratio of alt-RAS axis metabolites to PRA-S (ALT-S). We applied mixed linear models to assess how PRA-S and ACE/ACE2 concentrations affected ALT-S, ACE-S, and angiotensins II and 1-7. Median angiotensin I and II levels were higher with severe versus non-severe COVID-19 (angiotensin I: 86 versus 30 pmol/L, p < 0.01; angiotensin II: 114 versus 58 pmol/L, p < 0.05), demonstrating activation of classical RAS. The difference disappeared with analysis limited to patients not taking a RAS inhibitor (angiotensin I: 40 versus 31 pmol/L, p = 0.251; angiotensin II: 76 versus 99 pmol/L, p = 0.833). ALT-S in severe COVID-19 increased with time (days 1-6: 0.12; days 11-16: 0.22) and correlated with ACE2 concentration (r = 0.831). ACE-S was lower in severe versus non-severe COVID-19 (1.6 versus 2.6; p < 0.001), but ACE concentrations were similar between groups and correlated weakly with ACE-S (r = 0.232). ACE2 and ACE-S trajectories in severe COVID-19, however, did not differ between survivors and non-survivors. Overall RAS alteration in severe COVID-19 resembled severity of disease-matched patients with influenza. In mixed linear models, renin activity most strongly predicted angiotensin II and 1-7 levels. ACE2 also predicted angiotensin 1-7 levels and ALT-S. No single factor or the combined model, however, could fully explain ACE-S. ACE2 and ACE-S trajectories in severe COVID-19 did not differ between survivors and non-survivors. In conclusion, angiotensin II was elevated in severe COVID-19 but was markedly influenced by RAS inhibitors and driven by overall RAS activation. ACE-S was significantly lower with severe COVID-19 and did not correlate with ACE concentrations. A shift to the alt-RAS axis because of increased ACE2 could partially explain the relative reduction in angiotensin II levels.
© 2022. The Author(s).
Conflict of interest statement
Marko Poglitsch and Oliver Domenig are employed by Attoquant Diagnostics, Vienna/Austria, a company that received payments for RAS-Fingerprint and ACE2 measurements. None of the other co-authors declared a competing interest related to the present manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

