The nexus between RNA-binding proteins and their effectors
- PMID: 36418462
- PMCID: PMC10714665
- DOI: 10.1038/s41576-022-00550-0
The nexus between RNA-binding proteins and their effectors
Abstract
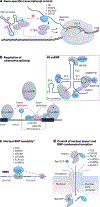
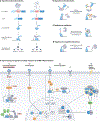
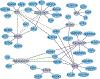
RNA-binding proteins (RBPs) regulate essentially every event in the lifetime of an RNA molecule, from its production to its destruction. Whereas much has been learned about RNA sequence specificity and general functions of individual RBPs, the ways in which numerous RBPs instruct a much smaller number of effector molecules, that is, the core engines of RNA processing, as to where, when and how to act remain largely speculative. Here, we survey the known modes of communication between RBPs and their effectors with a particular focus on converging RBP-effector interactions and their roles in reducing the complexity of RNA networks. We discern the emerging unifying principles and discuss their utility in our understanding of RBP function, regulation of biological processes and contribution to human disease.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures