Glycobiology of rheumatic diseases
- PMID: 36418483
- PMCID: PMC9684870
- DOI: 10.1038/s41584-022-00867-4
Glycobiology of rheumatic diseases
Erratum in
-
Author Correction: Glycobiology of rheumatic diseases.Nat Rev Rheumatol. 2023 Apr;19(4):253. doi: 10.1038/s41584-023-00922-8. Nat Rev Rheumatol. 2023. PMID: 36823186 Free PMC article. No abstract available.
Abstract
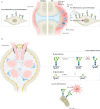
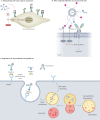
Glycosylation has a profound influence on protein activity and cell biology through a variety of mechanisms, such as protein stability, receptor interactions and signal transduction. In many rheumatic diseases, a shift in protein glycosylation occurs, and is associated with inflammatory processes and disease progression. For example, the Fc-glycan composition on (auto)antibodies is associated with disease activity, and the presence of additional glycans in the antigen-binding domains of some autoreactive B cell receptors can affect B cell activation. In addition, changes in synovial fibroblast cell-surface glycosylation can alter the synovial microenvironment and are associated with an altered inflammatory state and disease activity in rheumatoid arthritis. The development of our understanding of the role of glycosylation of plasma proteins (particularly (auto)antibodies), cells and tissues in rheumatic pathological conditions suggests that glycosylation-based interventions could be used in the treatment of these diseases.
© 2022. Springer Nature Limited.
Conflict of interest statement
R.E.M.T. and T.W.J.H. are mentioned inventors on a patent application on ACPA-IgG V-domain glycosylation. The remaining authors declare no competing interests.
Figures


References
-
- Jefferis R. Recombinant proteins and monoclonal antibodies. Adv. Biochem. Eng. Biotechnol. 2021;175:281–318. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

