Does the toxicity of endocrine therapy persist into long-term survivorship?: Patient-reported outcome results from a follow-up study beyond a 10-year-survival
- PMID: 36418518
- PMCID: PMC10036266
- DOI: 10.1007/s10549-022-06808-9
Does the toxicity of endocrine therapy persist into long-term survivorship?: Patient-reported outcome results from a follow-up study beyond a 10-year-survival
Abstract
Background: Endocrine treatment (ET) is a highly effective breast cancer treatment but can distinctly impair breast cancer patients' quality of life (QOL). In a patient-reported outcome (PROs) study conducted by the authors in 2011, patients reported higher ET-induced symptom levels than known from the registration trials, and was underestimated. Based on these study results, we investigated the long-term sequelae of ET reported by breast cancer survivors (BCS) in a follow-up study conducted 5-10 years after an earlier assessment.
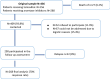
Methods: BCS who had participated in the earlier study (n = 436) were approached for study participation either at one of their routine follow-up appointments or via mail; consenting patients were asked to completed the same PRO assessment used in the original study (FACT-B + ES). BCS with relapse/ progressive disease were excluded from the analysis. We compared long-term endocrine symptomatology and overall QOL outcome (i.e. FACT-G and -ES sum score).
Results: A final sample of 268 BCS was included in the analysis. BCS reported a significant improvement of the overall endocrine symptomatology (baseline mean = 59 vs. follow-up mean = 62, p < 0.001), physical (baseline = 23.9 mean vs. follow-up mean = 24.8, p < 0.01) and functional well-being (baseline mean = 21.7 vs. follow-up mean = 22.7, p = 0.013) and overall QOL (mean baseline = 88.3 vs. mean follow-up = 90.9, p = 0.011). However, the prevalence of particular symptoms, well-known to be ET induced, did not change over time such as joint pain (baseline = 45.5% vs. 44.2%, n.s. difference), lack of energy (36.4% vs 33.8%, n.s. difference), weight gain (36.8% vs. 33.9%, n.s. difference) or vaginal dryness (30.2% vs. 33%, n.s. difference) and the proportion reporting lack of interest in sex increased (40.4% vs. 48.7%, p < 0.05).
Conclusion: Presented results indicate that BCS recover well in terms of overall endocrine symptomatology and quality of life but experience some clinically relevant and unfavorable ET-related long-term effects.
Keywords: Breast cancer; Endocrine therapy; Long-term breast cancer survivors; Patient-reported outcomes; Quality of life; Toxicity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Figures
References
-
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R, Early Breast Cancer Trialists' Collaborative G Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. - DOI - PMC - PubMed
-
- Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous