Subcutaneous versus transvenous implantable cardioverter-defibrillator among drug-induced type-1 ECG pattern Brugada syndrome: a propensity score matching analysis from IBRYD study
- PMID: 36418560
- PMCID: PMC10085956
- DOI: 10.1007/s00380-022-02204-x
Subcutaneous versus transvenous implantable cardioverter-defibrillator among drug-induced type-1 ECG pattern Brugada syndrome: a propensity score matching analysis from IBRYD study
Erratum in
-
Correction to: Subcutaneous versus transvenous implantable cardioverter-defibrillator among drug-induced type-1 ECG pattern Brugada syndrome: a propensity score matching analysis from IBRYD study.Heart Vessels. 2023 May;38(5):689-690. doi: 10.1007/s00380-022-02228-3. Heart Vessels. 2023. PMID: 36607387 Free PMC article. No abstract available.
Abstract
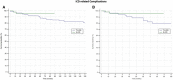
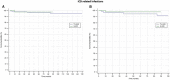
No real-world data are available about the complications rate in drug-induced type 1 Brugada Syndrome (BrS) patients with an implantable cardioverter-defibrillator (ICD). Aim of our study is to compare the device-related complications, infections, and inappropriate therapies among drug-induced type 1 BrS patients with transvenous- ICD (TV-ICD) versus subcutaneous-ICD (S-ICD). Data for this study were sourced from the IBRYD (Italian BRugada sYnDrome) registry which includes 619 drug-induced type-1 BrS patients followed at 20 Italian tertiary referral hospitals. For the present analysis, we selected 258 consecutive BrS patients implanted with ICD. 198 patients (76.7%) received a TV-ICD, while 60 a S-ICD (23.4%). And were followed-up for a median time of 84.3 [46.5-147] months. ICD inappropriate therapies were experienced by 16 patients (6.2%). 14 patients (7.1%) in the TVICD group and 2 patients (3.3%) in S-ICD group (log-rank P = 0.64). ICD-related complications occurred in 31 patients (12%); 29 (14.6%) in TV-ICD group and 2 (3.3%) in S-ICD group (log-rank P = 0.41). ICD-related infections occurred in 10 patients (3.88%); 9 (4.5%) in TV-ICD group and 1 (1.8%) in S-ICD group (log-rank P = 0.80). After balancing for potential confounders using the propensity score matching technique, no differences were found in terms of clinical outcomes between the two groups. In a real-world setting of drug-induced type-1 BrS patients with ICD, no significant differences in inappropriate ICD therapies, device-related complications, and infections were shown among S-ICD vs TV-ICD. However, a reduction in lead-related complications was observed in the S-ICD group. In conclusion, our evidence suggests that S-ICD is at least non-inferior to TV-ICD in this population and may also reduce the risk of lead-related complications which can expose the patients to the necessity of lead extractions.
Keywords: Brugada syndrome; Drug-induced type 1 Brugada syndrome; ICD-related complication; ICD-related infection; Implantable cardioverter-defibrillator; Inappropriate shock; Subcutaneous cardioverter-defibrillator; Sudden cardiac death; Transvenous cardioverter-defibrillator.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
-
- Russo V, Pafundi PC, Caturano A, Dendramis G, Ghidini AO, Santobuono VE, Sciarra L, Notarstefano P, Rucco MA, Attena E, Floris R, Romeo E, Sarubbi B, Nigro G, D'Onofrio A, Calò L, Nesti M. Electrophysiological study prognostic value and long-term outcome in drug-induced type 1 Brugada syndrome: the IBRYD study. JACC Clin Electrophysiol. 2021;7(10):1264–1273. doi: 10.1016/j.jacep.2021.03.010. - DOI - PubMed
-
- Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the european society of cardiology (ESC)endorsed by: association for european paediatric and congenital cardiology (AEPC) Europace. 2015;17(11):1601–1687. - PubMed
-
- Conte G, Sieira J, Ciconte G, de Asmundis C, Chierchia GB, Baltogiannis G, Di Giovanni G, La Meir M, Wellens F, Czapla J, Wauters K, Levinstein M, Saitoh Y, Irfan G, Julià J, Pappaert G, Brugada P. Implantable cardioverter-defibrillator therapy in Brugada syndrome: a 20-year single-center experience. J Am Coll Cardiol. 2015;65:879–888. doi: 10.1016/j.jacc.2014.12.031. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

