Development of therapeutic antibodies for the treatment of diseases
- PMID: 36418786
- PMCID: PMC9684400
- DOI: 10.1186/s43556-022-00100-4
Development of therapeutic antibodies for the treatment of diseases
Abstract
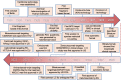
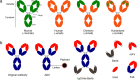
Since the first monoclonal antibody drug, muromonab-CD3, was approved for marketing in 1986, 165 antibody drugs have been approved or are under regulatory review worldwide. With the approval of new drugs for treating a wide range of diseases, including cancer and autoimmune and metabolic disorders, the therapeutic antibody drug market has experienced explosive growth. Monoclonal antibodies have been sought after by many biopharmaceutical companies and scientific research institutes due to their high specificity, strong targeting abilities, low toxicity, side effects, and high development success rate. The related industries and markets are growing rapidly, and therapeutic antibodies are one of the most important research and development areas in the field of biology and medicine. In recent years, great progress has been made in the key technologies and theoretical innovations provided by therapeutic antibodies, including antibody-drug conjugates, antibody-conjugated nuclides, bispecific antibodies, nanobodies, and other antibody analogs. Additionally, therapeutic antibodies can be combined with technologies used in other fields to create new cross-fields, such as chimeric antigen receptor T cells (CAR-T), CAR-natural killer cells (CAR-NK), and other cell therapy. This review summarizes the latest approved or in regulatory review therapeutic antibodies that have been approved or that are under regulatory review worldwide, as well as clinical research on these approaches and their development, and outlines antibody discovery strategies that have emerged during the development of therapeutic antibodies, such as hybridoma technology, phage display, preparation of fully human antibody from transgenic mice, single B-cell antibody technology, and artificial intelligence-assisted antibody discovery.
Keywords: AI-assisted antibody discovery; Antibody drugs; Immunotherapy; Phage display libraries; Single B-cell; Transgenic mice.
© 2022. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
